Granulomatous Polyangiitis (GPA) is a rare form of small vessel vasculitis characterized by the demonstration of necrotizing granulomatous inflammation histologically.
We report the case of a 62-years-old woman with symptoms of cough and significant loss of weight with radiological findings to support a left lower lobe lung mass. The patient was initially suspected to have metastatic lung malignancy until biopsy results suggested necrotizing granulomatous inflammation. A diagnosis of limited GPA was made when the cytoplasmic antineutrophil cytoplasmic antibodies, (c-ANCA) and antiproteinase-3 antibodies (anti-PR3) was elevated. The patient was treated with combination therapy of prenisolone (1 mg/kg) and methotrexate which induced clinical remission within weeks and radiological complete remission within 3 months.
However, as prednisolone was tapered down, there were suggestions of a relapse. Conclusion: The presentation of GPA can be highly variable and pose a diagnostic challenge to clinicians. In cases at risk for relapse, treatment should be continued for at least 18-24 months.
Granulomatous polyangitis, Wegener's granulomatosis, ANCA positive vasculitis, Small vessel vasculitis
GPA: Granulomatous Polyangiitis; c-ANCA: Cytoplasmic Antineutrophil Cytoplasmic Antibodies; anti PR3: Antiproteinase-3 Sntibodies; anti MPO: Myeloperoxidase Antibodies
Granulomatous Polyangiitis (GPA) formerly known as Wegener's Granulomatosis, is a rare form of small vessel vasculitis. It has no gender predominance and tends to present between ages of 40 to 55-years-old in more than two thirds of cases [1]. The aetiology of the disease remains unclear but there is a strong association with antineutrophil cytoplasmic antibodies (ANCAs) in immunofluorescence assays directed against proteinase-3 and myeloperoxidase [2]. Immune complex mediated small vessel vasculitis and microangiopathic in-situ thrombosis have been implicated in the pathogenesis but the hallmark of the disease is characterized by the identification of necrotising granulomatous inflammation [1,3].
Although any organ system can be involved, the involvement of the upper respiratory tract, lungs and kidneys are not uncommon. Pulmonary involvement of GPA is well described and is usually the most frequent organ of interest [4-6]. Early diagnosis is reliant on the high index of suspicion and awareness amongst clinicians as well as the crude ability to correlate clinical findings with diagnostic imaging, laboratory and histopathological results after exclusion of more common alternative diagnosis [1].
In this paper, we present a patient who was initially suspected to have metastatic malignancy based on imaging findings and once the patient was diagnosed with GPA, the therapeutic challenges we faced to keep the patient in remission.
Our patient, a 62-year-old woman with pre-existing marginally controlled Diabetes Mellitus presented to her physician in another health care facility with symptoms of chronic non-productive cough and loss of weight of more than 10 kg over 3 months period. 6 months prior to this, the patient had repeated ENT consultations for antibiotic refractory otitis media and sinusitis. Gradually, hearing on both ears diminished and the patient was found to have sensorineural deafness bilaterally. The patient became reliant on hearing aids ever since. Further detailed history revealed that the patient was a non-smoker and there was no occupational or environmental exposures to explain a chronic respiratory illness. The patient was only on standard diabetic treatment with oral hypoglycaemic agents and there was no encounters with elements of traditional healing. Physical examination findings were relatively unremarkable. Pulse rate was 100/min, blood pressure was 180/99 mmHg, respiratory rate was 16/min, body temperature was 37.2 ℃ and oxygen saturation (SO2) level was 97% on room air, as measured by pulse oxymetry. There was no nose deformity, skin rash, oral ulcers, joint tenderness or swelling to report.
Routine and specific laboratory test results findings are shown in Table 1. As demonstrated in Table 1, the patient had leukocytosis (neutrophil predominant), normochromic normocytic anaemia, thrombocytosis, elevated ESR, serum globulin and hypoalbuminaemia besides the suboptimal diabetic control. Chest radiograph (Figure 1) revealed an ill-defined lesion in the left lower zone. Chest computed tomography (CT) imaging (Figure 2) demonstrated multiple nodules including a 3.9 × 1.9 × 1.9 cm irregular mass in the posterobasal segment of the left lower lobe. There was no accompanying mediastinal lymphadenopathy or pleural effusion seen. Flexible bronchoscopy excluded any endobronchial lesions and broncho alveolar lavage (BAL) specimens were sent from the postero basal segment of the left lower lobe exclusively for tuberculosis (liquid media) culture and sensitivity which was eventually reported as negative. In order to rule out metastatic lung malignancy, the patient also underwent a percutaneous CT guided biopsy of the left lower lobe lung lesion. A total of 5 trucut biopsy specimens were taken of the left lower lobe lung lesion. Nodules besides the one in the left lower lobe were not suitable for percutaneous biopsy as they were smaller than 1 cm in size. Histopathological analysis revealed extensive necrotizing and suppurative granulomas (Figure 3A) which were poorly defined (Figure 3B) with the presence of multinucleated giant cells (Figure 3C). There were residual foci of alveolar spaces lined by reactive pneumocytes and interstitial inflammation. The inflammatory cells were mainly composed of neutrophils forming foci of microabscesses. No cellular dysplasia or malignant cells was seen. Acid fast bacilli staining was also negative. Simultaneously, the patient was found to have a positive c-ANCA with a high titre of anti-Proteinase-3 (anti-PR3) at 8.1 U/ml (normal range < 2.0 U/ml) and a negative anti Myeloperoxidase (anti-MPO).
Table 1: Routine and specific laboratory test results. View Table 1
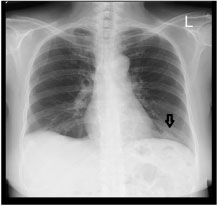 Figure 1: Chest radiograph on presentation, (black arrow) showing an ill-defined lesion in the left lower zone. View Figure 1
Figure 1: Chest radiograph on presentation, (black arrow) showing an ill-defined lesion in the left lower zone. View Figure 1
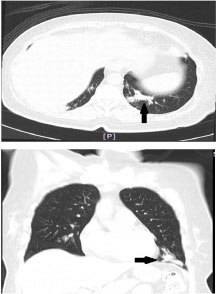 Figure 2: CT scan axial (above) and coronal (below) view, black arrows showing a mass measuring 3.9 × 1.9 × 1.9 (cm) in the posterobasal segment of the left lower lobe. View Figure 2
Figure 2: CT scan axial (above) and coronal (below) view, black arrows showing a mass measuring 3.9 × 1.9 × 1.9 (cm) in the posterobasal segment of the left lower lobe. View Figure 2
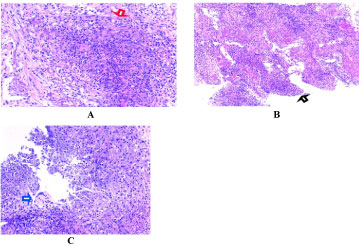 Figure 3: Showing histopathological slides of the left lower lobe mass (pre- treatment). Red arrowhead (A) Showing necrotizing and suppurative granuloma, black arrowhead; (B) Showing a poorly formed granuloma and blue arrowhead; (C) Showing multinucleated giant cells. View Figure 3
Figure 3: Showing histopathological slides of the left lower lobe mass (pre- treatment). Red arrowhead (A) Showing necrotizing and suppurative granuloma, black arrowhead; (B) Showing a poorly formed granuloma and blue arrowhead; (C) Showing multinucleated giant cells. View Figure 3
Following the diagnosis of limited GPA with pulmonary involvement preceded by the upper respiratory tract disease (sinusitis and ear involvement) which has been quiescent for months, the patient was counseled for systemic steroids and weekly oral methotrexate. The patient was initiated on prednisolone (1 mg/kg) 60 mg daily and oral methotrexate was started at 10 mg per week with adequate folic acid supplementation. Within the first few weeks of treatment, there was prompt resolution of respiratory symptoms and weight gain was observed. The patient was seen fortnightly and oral methotrexate was gradually uptitrated to a maximum dose of 20 mg per week after 8 weeks. Prednisolone was also gradually stepped down to (0.5 mg/kg), 30 mg daily within 12 weeks. There were no adverse drug reactions to report except the worsening of glycaemic control which was effortlessly optimised with lifestyle modifications and the adjustment of anti-diabetic medications. The patient also started to develop a mild Cushingoid habitus from the systemic steroid therapy which was expected.
At the end of 12 weeks, hearing loss was unaffected with treatment but besides that, the patient remained asymptomatic with a remarkable weight gain to the baseline state. Repeat CT thorax showed complete resolution of all lung masses and nodules. ANCA results were also negative for c-ANCA and anti-PR3 (0 U/mL). After 6 months, the prednisolone was further reduced to 10 mg daily and the methotrexate dose was kept at 20 mg per week. At this point c-ANCA remained negative with an anti PR3 titre of 1.2 U/mL. The rise in anti PR3 antibody prompted us to repeat another CT thorax which showed the re-emergence of the left lower lobe lung nodule (1.5 cm × 0.5 cm × 0.5 cm).
After multidisciplinary consensus, it was agreed due to the lack of major adverse events and the preservation of the overall wellbeing of the patient, her prednisolone was continued at 10 mg daily and methotrexate at 20 mg per week. Her ANCA panel remained negative over the next 6 months with negative c-ANCA and anti PR3 that ranged from 0.8 - 1 U/mL. The improvement in pulmonary symptoms was sustained and the disease was in remission for a period of 12 months. Her repeat CT thorax did not show any new nodules and progression of the residual nodule in the left lower lobe.
In our case report, we describe a lady in her early 60's with prolonged cough and significant loss of weight. Diagnostic imaging revealed a lung mass in the left lower lobe of the lung amongst many other nodules. While it's paramount that a diagnosis of either a primary or a metastatic lung malignancy be pursued with haste, tuberculosis is also a common possibility in this part of the world. However, the upper airway involvement (sinusitis, otitis media and hearing impairment) seen in our patient could have been an important clue to clinching the diagnosis of GPA much earlier and was possibly dismissed as a red herring. It has been reported that upper airway involvement can be present in patients with GPA months before manifestation of pulmonary or renal disease [7].
Whilst it makes sense that early recognition of a systemic disease and prompt exclusion of alternative diagnosis will eventually lead to a better outcome, the clinical picture of patients with GPA is often variable depending on which organ system is affected. Most patients report some mild non-specific constitutional symptoms before developing full blown disease in the likes of pulmonary hemorrhage, acute renal failure and cavitating pneumonia [5]. Therefore, the diagnostic challenges of GPA is not a walk in the park for clinicians and in most instances required a multidisciplinary approach [5]. Clinicians need to have a high index of suspicion for GPA in patients with diagnostic dilemmas so as not to miss the diagnosis as the disease most often have a rapidly progressive course and may surely lead to a fatal outcome if left untreated [8].
The detection of lung mass in our imaging led to the pursuit for tissue diagnosis in our patient. The discovery of necrotizing granulomatous inflammation from the lung mass biopsy made us suspect ANCA vasculitis and hence an ANCA panel was requested. Flexible bronchoscopy was performed to obtain a rapid diagnosis for pulmonary tuberculosis using the GeneXpert assay. In some exclusive instances, strongly positive c-ANCA and anti-PR3 results is sufficient to make the diagnosis of GPA without biopsy [5]. Although the positivity of these tests approach 90% in generalized disease, for limited disease ANCA can be undetectable in 20% of cases [2]. Hence, although it not compulsory to be pursue histopathological in all cases, but it surely is desirable as part of a comprehensive evaluation [2]. In our patient, despite her having limited disease, her c-ANCA and anti-PR3 titre was strongly positive indicating that her disease was highly active. This may have facilitated her diagnosis but certainly it suggested the need for a robust and aggressive treatment approach.
The current standard of care for GPA includes high dose corticosteroids combined with either cyclophosphamide or rituximab. For limited GPA, methotrexate combined with prednisolone is an option that led to a favorable outcome [9]. The preference of alternatives compared to cyclophosphamide was inevitable as the latter increased susceptibility to infection in 10% of cases [1]. However, the usage of combination therapy had revolutionized mortality rates from more than 90% when untreated to an acceptable range of 4-13% [8,9].
Universally, treatment is advocated to be continued for a minimal period of 18 months but shorter treatment regimens have also been suggested to minimize risks of infection [1,10]. Majority of patients achieve remission within 3-6 months with conventional treatment but in the real world, nearly half of them developed relapse when treatment is de-escalated or switched off [11]. In cases with high risk of relapse, treatment can be extended up to 24 months [1]. Although real practice, c-ANCA/anti PR3 titre is used to predict the risk of relapse in such patients, but over-realiance on this parameter is greatly disputed [12].
The histopathological findings in our patient transformed her bleak prognosis into a treatable and potentially curable disease. Although the biopsy results were not specific for GPA as other forms of small vessel vasculitis could also have similar histopathological appearance, correlation with the raised c-ANCA levels made the likelihood of GPA in this case to be diagnostic. Once the diagnosis of GPA was made, she responded well to initial treatment which induced remission rapidly within a matter of weeks. Although remission was sustained for at least a year, there were instances which suggested a possibility of relapse if treatment was further de-escalated. For that reason, low dose prednisolone and maintenance methotrexate will be continued for at least 18 months or even 24 months if the need is sustained.
None Declared.
The authors would like to thank the Director General of Health Malaysia for his permission to publish this article.