Pulmonary diseases associated with parasitic infections of the lung are unusual. However, the rise in immunosuppressive conditions such as HIV/AIDS, use of antineoplastic agents and post-transplant medications as well as the phenomenon of globalization and migration, has raised its prevalence both in immunocompromised and immunocompetent individuals. Tropical pulmonary eosinophilia (TPE) is a neglected tropical disease of predominantly filarial endemic countries caused by a hypersensitivity response to antigens from microfilariae of Wuchereria bancrofti and Brugia malayi. The disease affects less than 1% of patients with lymphatic filariasis, mainly young adult males. It has a slow onset of several months with respiratory symptoms mainly fever, cough, dyspnea and wheeze. The current criteria for the diagnosis of TPE include history supportive of exposure to lymphatic filariasis, nocturnal cough and dyspnea, pulmonary infiltrates in chest x-ray, elevated peripheral blood eosinophils and serum IgE levels, increased titers of anti-filarial antibodies and clinical response to diethylcarbamazine which is the standard treatment. When treated, the majority of patients recover clinically and functionally, however, a small group of patients develops features compatible with pulmonary interstitial fibrosis which encompasses a worse prognosis and ultimately a premature death. More research is necessary to understand the immunopathology of this disease and consequently improve our tools for early diagnosis and timely treatment.
Tropical pulmonary eosinophilia, Microfilariae, Diethylcarbamazine, Lung function, Radiology
In the twenty-first century, parasitic diseases still represent an important cause of morbidity, especially in tropical countries. Lung involvement is not common except in patients with immunosuppressive conditions such as malnutrition, autoimmune diseases, cancer and HIV infection. Additionally, an increase in the prevalence of these diseases has also been observed in developed countries mostly due to globalization and migration, hence the importance of their early recognition and timely treatment.
Lymphatic filariasis is a neglected tropical disease caused by nematodes of the Filaroidea family, the most important in humans being Wuchereria bancrofti (Figure 1a), Brugia malayi (Figure 1b) and Brugia timori. One-third of the cases of W. bancrofti filariasis have been reported in Africa, another third in India and the rest in South Asia, Pacific Rim and parts of Central and South America. Brugia species are reported to be present in South and Southeast Asia, in particular China, India, Indonesia, Malaysia, Timor, Sri Lanka, Thailand and Vietnam [1]. Elephantiasis is the most common clinical manifestation of the disease which results from obstruction of lymphatic vessels and the consequent lymphedema. In the year 2000, there were more than 120 million people infected with these mosquito-transmitted parasites and there are now more than 947 million people in 54 countries at risk requiring prophylactic treatment to stop the spread of the disease [2]. The World Health Organization has launched the Global Programme to Eliminate Lymphatic Filariasis (GPELF) with the aim of global elimination of lymphatic filariasis as a public health problem by the year 2020. The strategy behind this program includes large scale preventing chemotherapy with albendazole + ivermectin or diethylcarbamazine citrate annually to high-risk populations as well as morbidity management and disability prevention activities.
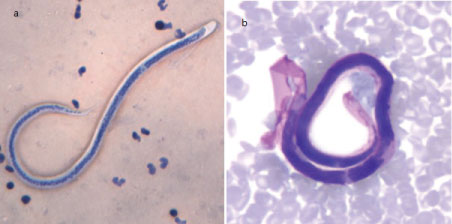 Figure 1: a) Microfilaria of W. bancrofti in a thick blood smear stained with Giemsa; b) Microfilaria of B. malayi in a thin blood smear stained with Giemsa. View Figure 1
Figure 1: a) Microfilaria of W. bancrofti in a thick blood smear stained with Giemsa; b) Microfilaria of B. malayi in a thin blood smear stained with Giemsa. View Figure 1
The life cycle of both types of filarias is very similar. In both cases, adult helminths are responsible for the clinical manifestations of lymphatic filariasis. Once the vector insect bites (the vectors include mosquitoes from the genera Anopheles, Culex, Aedes and Mansonia), third-stage larvae migrate through the skin into the cutaneous lymphatic vessels. The larvae begin their maturation process within the lymphatic system taking approximately 6 to 12 months to become adults. The resulting female larvae measure 80 to 110 mm in length and 0.24 to 0.30 mm in diameter while males measure 40 mm in length and 0.1 mm in diameter (adult Brugia malayi larvae are smaller; females measure 43 to 55 mm in length and 130 to 170 μm in width and males 13 to 23 mm in length and 70 to 80 μm in diameter). The adult forms migrate to the lymph nodes where they can remain for 5 to 10 years. The copulation of adult larvae results in microfilariae measuring 244 to 296 μm in length and 7.5 to 10 μm in width (in the case of Brugia malayi, the microfilariae measure 177 to 230 μm in length and 5 to 7 μm in width). These parasites are released into the blood with nocturnal periodicity, having an average life span of 3 months to 3 years. Once an insect bite occurs in a person with microfilariae in the blood, the parasite loses its cover and migrates to the wall of the proventriculus and the cardiac portion of the midgut until reaching the thoracic muscles. There, microfilariae develop into first-degree larvae and follow their evolution to third-degree larvae. Third-degree larvae migrate through the hemocoel into the proboscis of the mosquito and are kept there until a new bite and consequent infestation of a new host occurs [3,4].
 Figure 2: Life cycle of W. bancrofti and B. malayi. View Figure 2
Figure 2: Life cycle of W. bancrofti and B. malayi. View Figure 2
Tropical pulmonary eosinophilia (TPE) is the result of a hypersensitivity reaction to the microfilariae of Wuchereria bancrofti and Brugia malayi trapped in the pulmonary microcirculation. As a disease, it was first described in 1943 in India by Weingarten in patients with suspected tuberculosis who had "spasmodic bronchitis", eosinophilia and fine spotting on chest radiography [5].
TPE occurs in < 1% of patients with lymphatic filariasis, particularly in young adult males, with a male to female ratio of 4:1. Although it primarily affects the lung, approximately 7% of the patients show extrapulmonary and extralymphatic manifestations [6]. In the natural history of the disease, pulmonary pathological findings include 1) acute eosinophilic infiltration in the form of eosinophilic bronchopneumonia (Figure 3), 2) eosinophilic abscesses or a mixed cellular infiltrate (eosinophils and histiocytes) which appear 6 months to 2 years after infection and 3) in some chronic cases, the development of interstitial, peribronchial and perivascular fibrosis associated with the presence of histiocytes. [7].
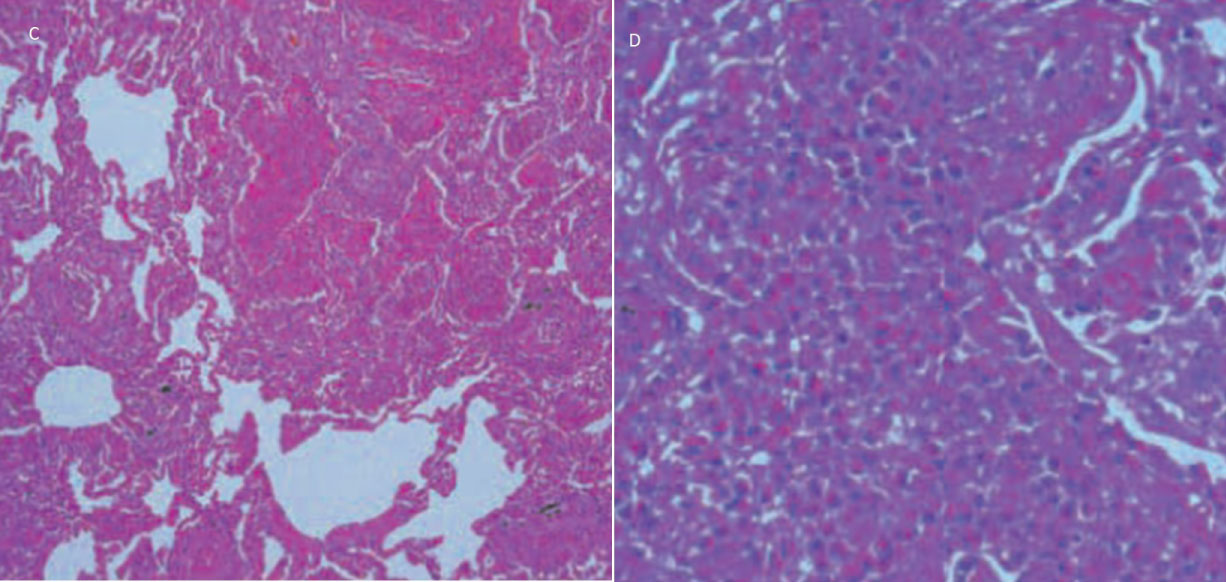 Figure 3: Haematoxylin and eosin stain low (C, 200X) and high (D, 400X) magnification views of lung biopsy specimen showing eosinophilic infiltration of alveolar spaces [82]. View Figure 3
Figure 3: Haematoxylin and eosin stain low (C, 200X) and high (D, 400X) magnification views of lung biopsy specimen showing eosinophilic infiltration of alveolar spaces [82]. View Figure 3
The exact pathogenesis of TPE is still unclear. Eosinophils are immune cells necessary for filarial nematode clearance [8-10]. Eosinophilic granule proteins such as eosinophil cationic protein (ECP), eosinophil-derived neurotoxin (EDN), major basic protein 1 (MBP-1) and eosinophil peroxidase (EPO) show helminthotoxic activity in vitro [11,12], however, in vivo studies demonstrate that these proteins are not sufficient for microfilariae clearance and that the downstream effects of eosinophil-induced cytokine responses are probably responsible, in part, for parasite clearance [10,13]. Sharma, et al. in a mouse model of TPE, demonstrated that lung eosinophils upregulate their levels of Ficolin-A, a lectin molecule associated with the activation of the complement system which participates in the process of parasite clearance [14]. Opsonization by antifilarial antibodies is also involved in microfilariae clearance. In some susceptible individuals, the presence of trapped microfilariae in the pulmonary circulation [15,16] or their antigenic products [17,18] generate an overactive systemic and compartmentalized pulmonary TH2 response characterized by elevated levels of IL-4, IL-5, polyclonal and filarial-specific IgE and IgG antibodies and massive pulmonary eosinophilia [19,20]. In contrast to patients who develop TPE, most individuals who live in filarial-endemic regions and who often are subject to recurrent filarial infections usually show suppressed immune responses and minimal clinical symptoms [21]. Eosinophils can also have damaging effects on the host during parasite infection particularly in the lung [10,22,23]. In patients with acute TPE, eosinophils are found to be 12-fold more concentrated in the lower respiratory tract and persistently activated and degranulated [21]. O'Bryan, et al. have shown that eosinophil-derived neurotoxin is selectively increased both systemically and in the lungs of individuals with TPE [11]. EDN is an eosinophil-associated RNase with a prominent role in host defense promoting leukocyte activation, migration, and chemotaxis [24]. Additionally, EDN has a direct damaging effect on the airway epithelium. This RNase also participates in the immunopathogenesis of other eosinophil-associated diseases such as asthma [25-27], cow's milk allergy [28] and eosinophilic esophagitis [29]. In conclusion, the precise role of eosinophils in the development of TPE is still largely unknown.
The Bm22-25, a major allergen of the L3 infective stage larvae of Brugia malayi, has been shown to stimulate T cell proliferation and IgE production both in vitro and in vivo [30]. Moreover, filarial B. malayi γ-glutamyl transpeptidase, a protein which shows molecular mimicry with Bm22-25 and is homologous to the mammalian γ-GT, is able to induce autoimmunity against human γ-GT present in lung epithelial cells [31]. Taken together, these findings show that BM22-25 and/or filarial B. malayi γ-GT might be involved in the pathogenesis of TPE. In mice, the administration of recombinant B. malayi γ-GT which induces a massive peribronchial, perivascular and alveolar inflammatory infiltration associated with the production of IFNγ, Il-4 and IgG2a/IgG3 antibodies [32] supports the idea that probably not only IgE-mediated Type I hypersensitivity responses but also Type III (immune complex) reactions may contribute to the pathogenesis of this disease.
Some argue that oxidants may play a role in both the acute and chronic forms of TPE. One year after patients were treated with standard DEC therapy, Rom, et al. showed that inflammatory cells of the lower respiratory tract still released exaggerated amounts of superoxide anion and hydrogen peroxide [33]. In these patients, a short oral course of prednisone treatment diminished the production of oxygen radicals from cells obtained by bronchoalveolar lavage [34]. However, the exact role of oxidants in this disease requires further analysis.
Patients with TPE usually present with nonspecific systemic signs such as fever, weight loss, and malaise associated with predominant nocturnal respiratory symptoms that include dry cough, dyspnea, wheeze and chest pain. Physical examination reveals scattered rhonchi and rales and, in some cases, extrapulmonary manifestations such as lymphadenopathy and hepatosplenomegaly. Sputum is usually absent but when present is viscous and mucoid with significant eosinophilia. Leukocytosis in peripheral blood is common with a predominance of eosinophils that can be as high as 50,000 to 80,000 cells/cubic millimeter [35,36]. Microfilariae are usually not observable in peripheral blood. Erythrocyte sedimentation rate is elevated in most cases and usually, returns to normal values after treatment. Serum IgE and IgG levels are elevated; Neva, et al. has reported patients with serum IgE levels as high as 2355 ng/ml with significant reduction but not total normalization after treatment [37]. Both IgE and IgG antibodies are cross-reactive with other helminth antigens and since most individuals come from endemic areas, it's important to discard other helminth infections such as Ascaris or Strongyloides infection which can be seen in up to 20% of patients [38,39]. Some patients may show arterial hypoxemia probably due to ventilation/perfusion mismatch [40]. In advanced cases, TPE can present with signs of pulmonary arterial hypertension due to the restrictive ventilatory defect and/or continued embolization of dead microfilariae in the lung capillaries [41,42]. Other cardiovascular manifestations that may be present include electrocardiographic abnormalities, pericarditis and pericardial effusion [43,44].
Differentiation of TPE from other parasitic helminth infections that present with eosinophilia and pulmonary symptoms such as strongyloidiasis, ascariasis, paragonimiasis, ancylostomiasis, toxocariasis and schistosomiasis [45,46] and gastrointestinal helminth infections that manifest as TPE-like syndrome [47] is important. The current diagnostic criteria for TPE include history supportive of exposure to lymphatic filariasis in an endemic area, nocturnal cough and dyspnea, pulmonary infiltrates in chest x-ray, elevated peripheral blood eosinophils (> 3 × 109 cells/L), elevated serum IgE levels (> 1000 kU/L), increased titers of filarial specific serum IgG1, IgG4 and IgE antibodies and clinical response to diethylcarbamazine [48]. An Og4C3 and a Bm-SXP-1 antigen ELISA test has been developed and shows high sensitivity and specifity to detect W. bancrofti [49] and B. malayi infections [50], respectively. Also, RT-PCR assays have proven to be equally sensitive to conventional parasitological methods for detection of these parasites [51]. However, assays for detection of circulating filarial antigens as well as molecular diagnostic methods are currently not approved by the US Food and Drug Administration. In the differential diagnosis, non-infectious causes of eosinophilia are to be considered and these include drug hypersensitivity, asthma, allergic rhinitis, allergic bronchopulmonary aspergillosis, acute eosinophilic leukemia and myeloproliferative disorders [52].
The most common chest radiology finding is bilateral fine diffuse reticulonodular opacities in the middle and lower lung zones [53] (Figure 4). Some patients may show fine mottling similar to the radiological findings of miliary tuberculosis (Figure 5). However, 20% of patients have normal chest radiograph [39]. In advanced disease, honeycomb changes typical of pulmonary fibrosis may be seen. Other unusual findings include consolidation [54], lung masses [55], cavitation [56], hydropneumothorax [57] and pleural effusion [58]. CT scan of the thorax show reticulonodular pulmonary opacities (Figure 6), bronchiectasis, air trapping and mediastinal lymphadenopathy [59,60]. Chest radiology findings usually disappear with treatment but up to 40% of patients still show radiographic abnormalities after one month of treatment [61].
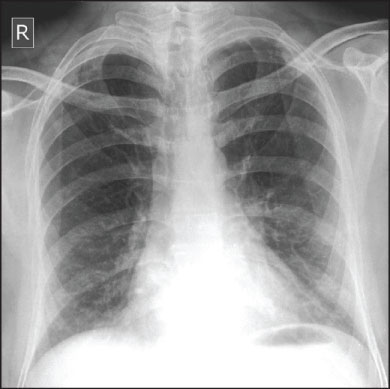 Figure 4: Chest X-ray showing diffuse reticular nodular opacities [83]. View Figure 4
Figure 4: Chest X-ray showing diffuse reticular nodular opacities [83]. View Figure 4
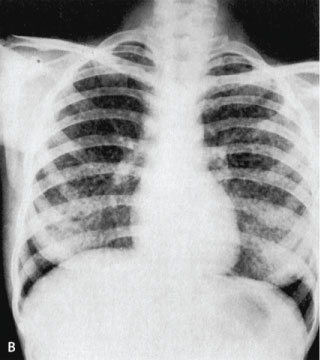 Figure 5: Chest X-ray showing bilateral miliary nodules [84]. View Figure 5
Figure 5: Chest X-ray showing bilateral miliary nodules [84]. View Figure 5
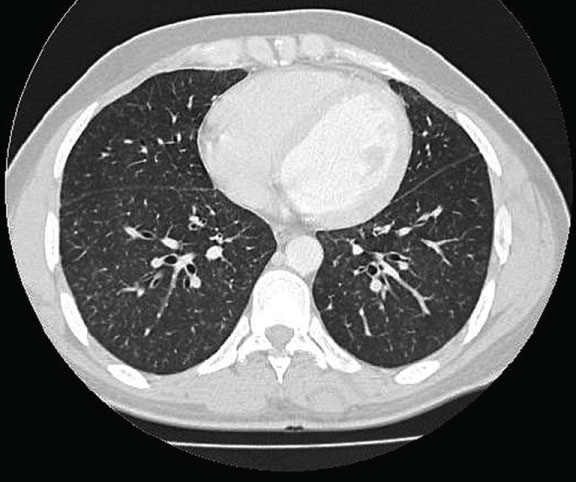 Figure 6: HRCT showing bilateral diffuse randomly distributed micronodules shadows [85]. View Figure 6
Figure 6: HRCT showing bilateral diffuse randomly distributed micronodules shadows [85]. View Figure 6
In acute TPE, a mild to moderate obstructive ventilatory pattern is usually observed [62]. In more advanced cases, pulmonary function tests usually show a restrictive pattern but, in approximately 30% of patients, a superimposed irreversible obstructive ventilatory defect can also be present [63,64]. Some persisting abnormalities in patients treated with DEC include a slight decrease in forced expiratory volume and a reduction in single breath transfer factor which is probably due to a reduced diffusion capacity for carbon monoxide via a reduction in the number of alveoli [65,66]. Other parameters such as peak expiratory flow rate and forced expiratory flow which are diminished in patients with untreated TPE can continue to be diminished after DEC treatment [67]. Surprisingly, Vijayan, et al. observed a negative correlation between the numbers of inflammatory cells in the bronchoalveolar lavage of patients with untreated TPE and lung function which suggests that there is an apparent dissociation between the presence of inflammatory cells in the lower respiratory tract and their impact over the pathophysiology of the lung; however, in this study eosinophils tend to be less detrimental to lung function than other inflammatory cells such as macrophages and lymphocytes [68].
Diethylcarbamazine citrate (N,N-diethyl-4-methyl-1-piperazine carboxamide; DEC) is an antihelminthic that has been used since 1947 for the treatment of filariasis, including lymphatic filariasis and TPE. The World Health Organization recommends a standard therapy of oral DEC at a dose of 6 mg/Kg divided TID for 21 days [44]. The rapid response to DEC treatment is characteristic of this disease. DEC treatment has shown to rapidly reduce eosinophil lung and blood count and titers of specific antifilarial IgE and IgG4 [14,69]. This effect may be in part explained by DEC's ability to directly suppress IL-5 dependent eosinophilopoiesis by iNOS and CD95L dependent mechanisms [70]. In vitro and in vivo, DEC has been shown to have direct microfilaricidal activity [71,72]. DEC interferes with arachidonic acid metabolism including both the cyclooxygenase and lipoxygenase pathways in microfilariae and hosts endothelial cells. Additionally, inducible nitric oxide is essential for the sequestration of microfilariae by DEC [73-75]. DEC also stimulates neutrophil and eosinophil adherence which may explain partially its role in enhancing innate immune responses [76]. Cesbron, et al. have shown that the effect of DEC is mediated by platelets and is enhanced by filarial excretory antigen. The microfilaricidal effect is antibody-independent and involves the participation of free radicals [77].
A failure rate of 20-40% to DEC treatment has been reported. Possible causes include incomplete treatment (dosage and/or duration) or advanced disease. Several studies have shown persistent airway inflammation and diminished lung function despite standard DEC treatment [33,61,66,78]. In these cases, use of potent anti-inflammatory drugs such as corticosteroids have shown promising results [79,80]. Pulmonary strongyloidiasis should be ruled out since use of steroids in these cases can cause disseminated infection [81].
Diethylcarbamazine has little toxicity. Main side effects include headache, fever, and gastrointestinal manifestations. Use of DEC is contraindicated in patients with onchocerciasis due to the intense systemic reactions (hypotension, pruritus, fever, adenitis) attributed to the death of circulating microfilariae (Mazzotti reaction). Also, DEC should be carefully administered to patients with Loa loa microfilariae levels > 2,500/mm3 due to potential life-threating effects that include renal failure and meningoencephalopathy.
Tropical pulmonary eosinophilia is an occult form of filariasis which is the result of a hypersensitivity reaction to trapped microfilariae or the antigenic products of Wuchereria bancrofti and Brugia malayi present in the pulmonary microcirculation. This disease affects mainly young males from endemic countries, however, the rise in immunosuppressive conditions associated with increasing rates of traveling and migration are responsible for the emergence of cases in non-endemic countries. The disease affects initially the lung airways and is characterized by the development of eosinophilic alveolitis that may progress, in chronic cases, to interstitial, peribronchial and perivascular fibrosis. Diethylcarbamazine is the standard pharmacological treatment, however, in a group of patients, a persistence of airway inflammation and significant alterations of lung function may be seen despite treatment. Better diagnostic tests and pharmacological drugs are needed to minimize morbidity and avoid the development of interstitial pulmonary fibrosis which profoundly affects the quality of life of these patients.
The author does not have any conflict of interest associated with the content of this article.