Background: Lymphovascular space invasion (LVSI) predicts nodal involvement, progression-free survival, and overall survival in patients with endometrial cancer. Despite its importance, the current approach to determining LVSI relies on slide review, which has substantial variability across pathologists. This variability is clinically important because it may alter the recommendation for treatment with radiation. Our study investigates whether immunohistochemical counterstaining to mark the border of the lymphovascular space increases diagnostic agreement among pathologists in diagnosing LVSI.
Design: This is a single-center retrospective case series of 70 cases of endometrioid type endometrial adenocarcinoma diagnosed between May 1, 2005, and December 31, 2013. One representative block of each case was cut and stained with hematoxylin and eosin (H&E), reviewed, then stained with the endothelial marker CD31 and the lymphatic endothelium marker D2-40 and reviewed again. The slides were reviewed independently by six gynecologic pathologists. We quantified intra-rater reliability with Cohen's κ and inter-rater reliability with Krippendorff's α. We powered our study to detect a change in Krippendorff's α of 0.20.
Results: Counterstaining had no significant effect on inter-rater reliability of LVSI diagnosis. Krippendorff's α was 0.503 [0.351-0.631] without counterstaining and 0.469 [0.319-0.589]. Brackets denote the 95% confidence interval. Counterstaining made pathologists revise their diagnoses with the average intra-rater reliability being 0.68 [0.58-0.78]. The most common change was from indeterminate LVSI to negative LVSI, which occurred in 87% (14 ± 6) slides. Pathologists reclassified positive LVSI to negative in 41% (4.5 ± 0.5) slides.
Conclusions: In our study, counterstaining with CD-31 and D2-40 did not increase agreement among pathologists in determining LVSI but did decrease the number of slides pathologists identified as indeterminate LVSI. Our findings suggest that counterstaining decreases the number of indeterminate LVSI diagnoses but does not increase the reliability of LVSI assessment.
Endometrial cancer is the most common gynecologic malignancy in the United States. Over 60,000 cases are diagnosed each year. The most common type of endometrial cancer is endometrioid adenocarcinoma [1].
Endometrioid adenocarcinoma has a favorable prognosis because 70% of the time it is in the early stages. The 5-year survival rate is greater than 95% for patients with stage 1 endometrial cancer [2]. The diagnosis of endometroid adenocarcinoma is made histologically and staged surgically according to the International Federation of Gynecology and Obstetrics (FIGO) and American Joint Committee on Cancer/Tumor, Nodes, Metastasis (TNM) classification system [3]. Treatment is based on the stage and presence of prognostic factors, such as tumoral invasion of the lymphovascular space (LVSI). Surgery alone is curative for women with FIGO stage IA or lower cancer.
It is crucial to accurately identify LVSI because LVSI is an independent prognostic factor and strong predictor of recurrence and death [4]. LVSI is associated with a roughly 90% increased risk of lymph node metastasis and 60% greater chance of progression of disease despite treatment [4]. Patients with LVSI, tumoral invasion of the outer half of the myometrium or of the cervical stroma are at higher risk for recurrence and may benefit from adjuvant radiation therapy, but patients with fewer than three of those risk factors may not benefit [5,6].
The diagnosis of LVSI is currently made when a pathologist observes tumor cells within a lumen lined by endothelial cells or attachment of tumor cells to the vascular wall. Lymphatic invasion is most readily assessed in the myometrium adjacent to the tumor [7]. The assessment of LVSI has a high inter-observer variability, even across pathologists specialized in gynecologic malignancies [8]. Artifactual retraction of stroma around the tumor and displacement of tumor cells during dissection of the specimen may simulate lymphatic space invasion.
Immunohistochemical stains to emphasize the borders of the vascular space can help pathologists detect LVSI more reliably. Staining with CD31 and pancytokeratin, markers of epithelial cells and tumors derived from epithelial cells, doubled recognition of endometroid endometrial cancer [9]. Staining with von Willebrand’s factor to identify blood vessels decreased the predictive value of LVSI assessment [10].
In this study we determine whether immunohistochemical staining with CD31 and D2-40 increases agreement among pathologists in diagnosing LVSI. CD31, also called PECAM, is a marker for endothelial cells [11]. It is a component of endothelial intercellular junction. D2-40 is a monoclonal antibody against a glycoprotein found specifically in lymphatic endothelium.
This retrospective chart review was reviewed by the Institutional Review Board and granted exemption from full review. We identified 65 cases of endometrioid endometrial cancer diagnosed between May 1, 2005, and December 31, 2013. We excluded serous and clear cell subtypes. Seventy de-identified slides were selected by authors MM and TK from the 65 cases. Six board-certified pathologists read each slide, three gynecology oncology pathology specialists and three general pathologists. Each pathologist independently assessed for tumor, grade, and LVSI using their own criteria. No diagnostic guidelines were provided, as previously described by Harris, et al. (2008) [12]. The pathologists assessed for LVSI on each case on three separate occasions and recorded their interpretations as present, absent, or indeterminate. For the first session, the pathologists reviewed one H&E slide from each case. For the second session the pathologists reviewed the original H&E slide, one counterstained for D2-40, and one for CD31. For the final session, the pathologists were given the H&E slides. The pathologists were blinded to each other’s reading, the clinical history of the cases, and their previous interpretations. No markings were permitted to be made on the study slides. The blinding and circulation of the slides was carried out by a non-pathologist member of the study team. There was at least two weeks in between each session to minimize recall bias. Figure 1 summarizes the study design.
 Figure 1: CONSORT-style diagram of study flow.
View Figure 1
Figure 1: CONSORT-style diagram of study flow.
View Figure 1
Serial sections were cut to 4-micron thickness. Sections were stained with hematoxylin and eosin. Staining with CD31 and D2-40 was performed. The protocol used for CD31 staining employed the JC70 monoclonal antibody (PAb), Cell Marque-Ventana, Cat# 760-4378. For podoplanin (D2-40), Cell Marque-Ventana, Cat# 760-4395 was used. The secondary antibody was OmniMap anti-mouse HRP, Ventana, Cat# 760-4310. Staining was performed using the RUO Discovery Multimer V2 on the Discovery Ultra Staining Module. Figure 2 shows an example of LVSI through each stage of staining.
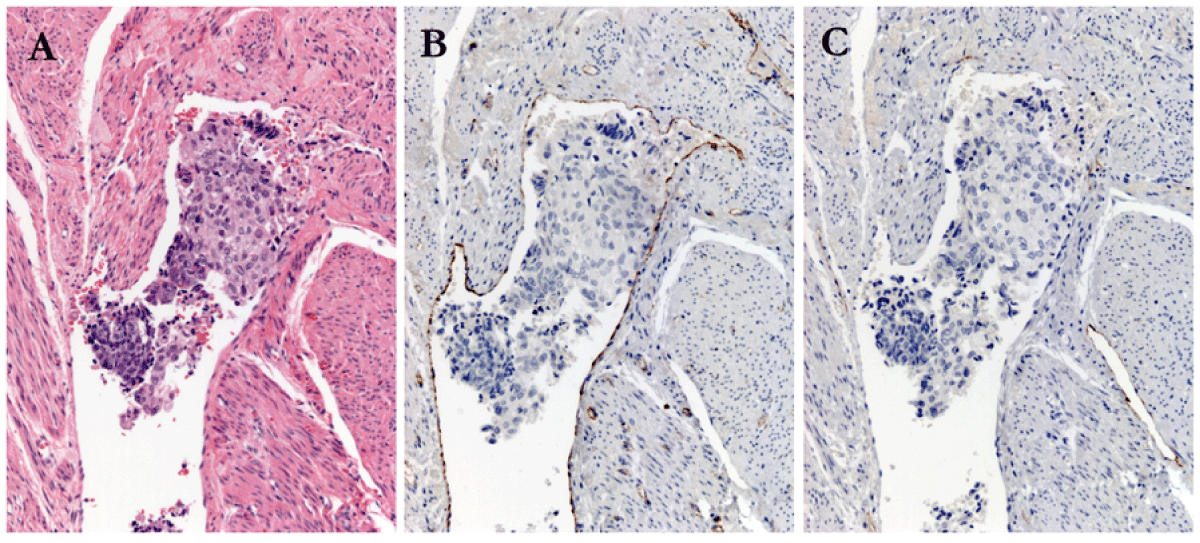 Figure 2: Positive Lympho-Vascular Invasion, case # 15, (A): Hematoxylin and Eosin 200X magnification; (B) CD 31 staining; and (C) D2-40 staining
View Figure 2
Figure 2: Positive Lympho-Vascular Invasion, case # 15, (A): Hematoxylin and Eosin 200X magnification; (B) CD 31 staining; and (C) D2-40 staining
View Figure 2
All analysis was performed in the Python programming language [13], using the pandas plugin [14] to tabulate the data and statsmodels to calculate Cohen’s κ and Krippendorff’s α [15]. Figures were made using the matplotlib plugin [16]. Our computer code is available at https://github.com/mac389/LVSI.
We chose a sample size of 70 slides to power the study to detect a 0.20 increase in Krippendorff’s α with a confidence interval of 0.1 and 80% power. We considered the smallest clinically meaningful change in Krippendorff’s α to be 0.20, which corresponds to increasing the agreement by one category, for example from minimal to moderate or moderate to substantial. We used Krippendorff’s formula to calculate the sample size [17]. For 6 raters and 3 response categories this requires 1,050 effective comparisons, corresponding to 70 slides. Each slide is rated by 6 pathologists, yielding 15 pairwise comparisons per slide.
Demographics of study population. The median patient age was 65. Figure 3 shows the distribution of ages. Figure 4 shows the distribution of self-identified ethnicities across these patients. To identify sampling biases in our data, we performed a multivariate regression of LVSI score against ethnicity and age. The 95% confidence intervals for both regression coefficients included zero.
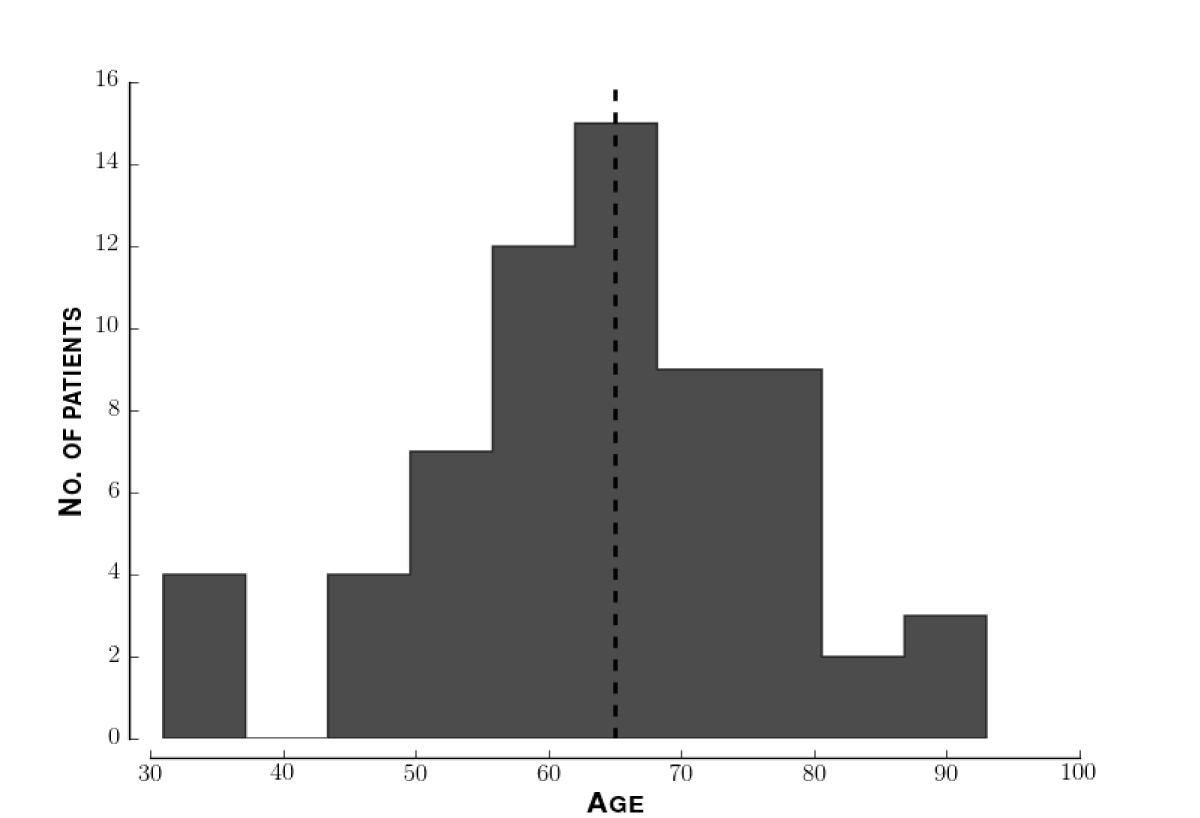 Figure 3: Distribution of ages of patients. Histogram of ages of all patients enrolled. The horizontal dotted line indicates the median age, 65.
View Figure 3
Figure 3: Distribution of ages of patients. Histogram of ages of all patients enrolled. The horizontal dotted line indicates the median age, 65.
View Figure 3
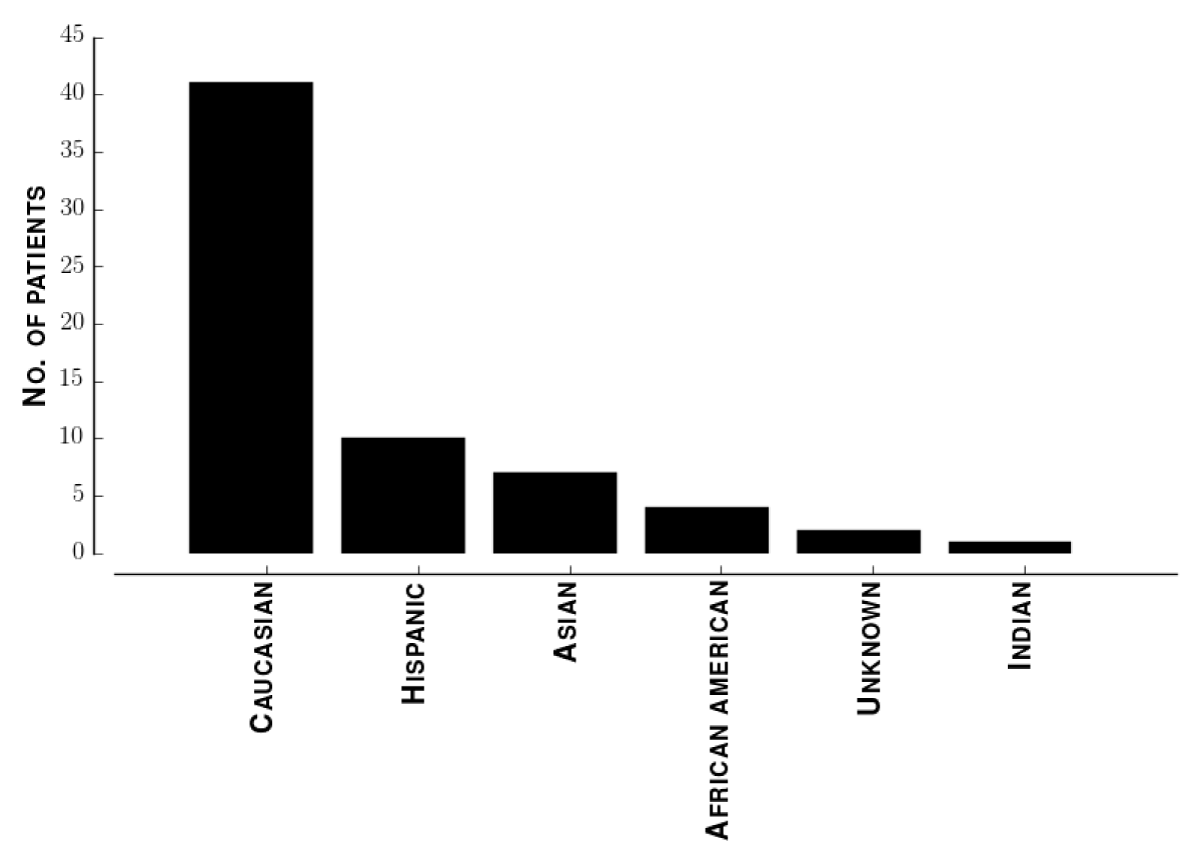 Figure 4: Distribution of ethnicities of patients. Histogram of ethnicities of all patients enrolled.
View Figure 4
Figure 4: Distribution of ethnicities of patients. Histogram of ethnicities of all patients enrolled.
View Figure 4
Inter-rater reliability. For identifying LVSI, the reliability was 0.503 [0.351-0.631] without counterstaining and 0.469 [0.319-0.589] with counterstaining, indicating fair agreement (Table 1). The difference between Krippendorff’s α with and without staining was not statistically significant (p = 0.491). The median intra-rater reliability α interquartile range was 0.68 ± 0.10, indicating that pathologists did change their assessment of LVSI after counterstaining (Table 2). The average inter-rater reliability was 0.86 ± 0.08 before counterstaining and 0.65 ± 0.12 after counterstaining, a statistically significant decrease (p = 0.02).
Table 1: Pathologists substantially agree on grading specimens, moderately agree on the presence of tumor and LVSI. Staining does not improve LVSI agreement, Krippendorf's α with 95% confidence interval in brackets. View Table 1
Table 2: Immunohistochemistry leads pathologists to change their assessment. Intra-rater reliability between LVSI ratings with and without immunohistochemical staining. View Table 2
To elucidate the revisions to ratings summarized by the intra-rater reliability we calculated the contingency table of ratings before and after IHC staining (Table 3). The most common change was from indeterminate LVSI to negative LVSI, which occurred in 87% of cases, 14 ± 6, and slides. Pathologists reclassified positive LVSI to no LVSI on average of 41% of the time, 4.5 ± 0.5 slides.
Table 3: After IHC staining, pathologists revise their readings, most frequently from indeterminate to no LVSI. Each cell shows the median number of cases that received the row rating before IHC staining and the column rating after IHC staining, expressed as median ± interquartile range. (+) denotes LVSI positive. (-) LVSI negative. (?) LVSI indeterminate. View Table 3
Table 4: Contingency table between histological grade of tumor and LVSI. Each cell shows the median number of cases that received the column rating by one pathologist and row rating by another pathologist's ± interquartile range. View Table 4
For tumor identification, the reliability was 0.54 [0.426-0.631] and for tumor grade, 0.700 [0.612-0.778], expressed as point estimate [95% confidence interval], indicating good agreement.
The main finding of this article is that immunohistochemical counterstaining with CD31 and D2-40 does not increase agreement among pathologists in assessing lymphovascular space invasion. A secondary finding is that counterstaining reduces the number of indeterminate cases, most frequently reclassified as negative. Thus, counterstaining may reduce the number of patients who needlessly receive radiation. This supports the current practice of using IHC only for indeterminate sections.
Despite compelling theory, staining with CD31 and D2-40, did not increase agreement among pathologists in determining LVSI in colorectal cancer [12], colorectal cancer with hepatic metastases [18], or breast adenocarcinoma [19]. Endothelial markers could increase the visibility of tumor mimics such as mucin pools or pseudoendothelial spaces, rendering the utility of IHC dependent on the pathologist’s ability to distinguish those from lymphovascular spaces.
The current standard for assessing LVSI is the pathologist’s reading. Thus, our study can determine whether counterstaining improves agreement but not accuracy. Cutting serial sections may confound assessment. Lympovascular spaces that are present on the first cut, may not be present on the subsequent cut, allowing both positive LVSI and negative LVSI to be accurate interpretations.
The question of how to increase uniformity of assessment of LVSI assumes that LVSI has prognostic utility. The evidence for LVSI predicting recurrence and 3- and 5-year survival comes from retrospective analyses [20-23]. These analyses convincingly demonstrate an association between LVSI and poorer outcomes, although the prognostic value found in those studies may reflect invasion of the myometrium [24,25].
It is not clear whether the changes pathologists made in response to immunostaining are accurate. There is no gold standard against which to compare the pathologist in the absence of unequivocal biomarkers except the readings of other pathologists. One confounder is that on cutting serial sections from the tissue block, as must be done on order to generate differently stained tissue sections, minute foci such as LVSI that are present on the first cut, may no longer be present on a subsequent cut. Hence a pathologist may make an accurate interpretation of the slide, and there will be an apparent lack of intra-observer concordance between the initial H&E slide and the immue-stained slide. The decrease in inter-rater reliability arises because pathologists differ in how they revise their diagnoses to incorporate information from IHC, which makes an appeal to consensus problematic.
The question of how to increase uniformity of assessment of invasion of lymphovascular space is also linked with the question of whether inter-rater reliability is an appropriate measurement. There are no accepted reference ranges for inter-rater reliability. A multi-center trial or meta-analysis to estimate the distribution of inter-rater reliabilities across a representative sample of gynecologic pathologists could establish such a range.