Pleural mesothelioma is aggressive, surgery is the principal treatment. Mesothelioma usually diagnosed in an advanced stage. The present study was conducted to study the circulating MiR-497-5p as a possible non-invasive molecular marker for the diagnosis of mesothelioma and its role in follow-up and prognosis.
MiR-497-5p was evaluated in Mesothelioma tissues and the plasma of patients and the plasma of healthy controls. MiR-497-5p was assessed in the plasma of patients before and after surgical excision of mesothelioma.
MiR-497-5p was downregulated in tumor tissues compared to normal tissues; it was also downregulated in the plasma of patients compared to the healthy controls. MiR-497-5p levels in the tissues and plasma were comparable. The plasma levels of MiR-497-5p were elevated in the patients after tumor excision. Downregulation was associated with adverse prognostic features.
MiR-497-5p acts as a tumor suppressor in mesothelioma. The circulating MiR-497-5p can be used as a possible non-invasive molecular marker for diagnosis and prognosis in mesothelioma patients.
Mesothelioma, Circulating MiR-497-5p, qRT-PCR, Diagnosis
Malignant pleural mesothelioma (MPM) is an uncommon disease, it is linked to asbestos and mineral fibers exposure, and its incidence is increasing [1]. The median survival is less than one year [2]. MPM is an aggressive tumor usually presented in an advanced stage [3].
MicroRNAs are short RNA 18-22 nucleotides that act as regulators of specific mRNA targets and involved in many biological functions and the pathogenesis of many diseases including malignancy [4]. Post-transcriptionally, they regulate the target gene negatively by either suppressing mRNA translation or enhancing the degradation of mRNAs [5]. They can function as an oncogene or as tumor suppressors by triggering one or maybe hundreds of mRNAs [6]. MicroRNAs can be secreted from all types cell to the extracellular fluid and transferred other body fluids like urine [7], blood [8], and serum [9]. About 10% of the discovered human MicroRNAs are detectable in plasma in a stabilized form [10]; this makes these small molecules ideal to act as molecular markers in cancer [11-13].
MiR-497HG human gene is located on chromosome 17p13.1, the first intron of the gene encodes MiR-497. In several types of malignant tumors, MiR-497-5p was found to be downregulated such as head and neck squamous cell carcinoma (HNSCC) [14], hepatocellular carcinoma (HCC) [15], pancreatic carcinoma [16], breast cancer [17], and colorectal carcinoma [18]. The functional role of MiR-497-5p is not fully explored in cancer, especially in MPM. To improve survival in MPM, we need to explore new molecular non-invasive markers for early diagnosis of MPM.
This study aims to study the circulating MiR-497-5p in plasma as a potential non-invasive molecular marker for early diagnosis, prognosis, and follow-up in MPM.
This study was carried out at Al Hadda Military Hospital, Taif, SA, between June 2016 and November 2019, the study was approved by Al Hadda hospital ethical committee. The study included 30 patients (13 males and 17 females) with pathologically proven malignant mesothelioma. The patient’s age ranged between 46 and 66 years. Twenty-two patients have good performance status. Written informed consent was obtained from all recruited patients. The performance status of the patients was assessed according to Zubord Scale [19] and patients were staged according to the latest version of the American Joint Committee of Cancer (AJCC) [20]. The patient's characteristics are presented in Table 1.
All patients underwent surgical excision; biopsies were obtained from tumor tissues and nearby pleural tissues (NNT) not invaded by the tumor. Tissue specimens were stored in liquid nitrogen at -80 °C until analysis.
2 ml of venous blood was withdrawn from all patients included before surgery and 2 weeks after tumor resection. Blood samples were also obtained from 30 normal healthy individuals. Samples centrifuged at 4 °C and 1200 g for 10 minutes in ethylenediaminetraacetic acid tubes to separate the plasma free nucleic acids. Centrifugation was repeated to remove cells and cell debris. The supernatant plasma was collected and stored at -80 °C in liquid nitrogen until analysis.
Total RNA was extracted from tissues and plasma by the miRNeasy Mini Kit (Qiagen, Hilden, Germany) guided by the manufacturer directions. The purified samples of RNA stored at -81 °C until use.
Synthesis of complementary DNA (cDNA): Reverse transcription of extracted RNA was done by iNtRON Biotechnology to synthesize cDNA according to the company guidelines.
RT-PCR: SYBR Green PCR Master Mix (Perfect Real Time; TaKaRa Biotechnology, Shiga, Japan) was used to perform the Quantitative real-time polymerase chain reaction (Q RT-PCR) according to the company guides by the Bio-Rad CFX96 sequence detection system (Bio-Rad Laboratories Inc.).
A 20 μl of amplification mixture contains 10 μl SYBR Green master mix, 5 μl cDNA, and 100 pmol/μl of the primers. The amplification was conducted by Stratagene Mx3005P-qPCR. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used for control. The primers sequence of primers was as follow:
GAPDH sense: 5'-GTCAACGGATTTGGTCTGTATT-3',
GAPDH reverse: 5'-AGTCTTCTGGGTGGCAGTGAT-3',
MiR-497-5p sense: 5’-GTCGTATCCAGTGCAGGGTCCGAGGT,
MiR-497-5p reverse: ATTCGCACTGGATACGACACCAAACA-3’.
All reactions were conducted in triplicate; MiR-497-5p was normalized to GAPDH. The reactions of qPCR consists of denaturation and polymerase activation for 2 minutes at 95 °C followed by denaturation for 15 seconds at 95 °C for 40 cycles then annealing and elongation for 1 minute at 58 °C. Gene expression was quantitated by the 2-ΔCt method [21].
Statistical analysis was performed using version 9.4 of the SAS software package (SAS Institute, Inc.; Cary, NC, USA).
We analyzed the expression of MiR-497-5p by qRT-PCR in mesothelioma and their nearby normal tissues. We found that the MiR-497-5p level in mesothelioma ranged between 0.241 and 1.841(2-ΔCt) and in NNT 1.92 and 4.62. The mean ± SD in mesothelioma was 1.1 ± 0.51 and in NNT 3.5 ± 0.7 (Figure 1A). This difference was statistically significant (t = 14.44, < 0.0001). A receiver operating characteristic (ROC) curve was generated to test the specificity of MiR-497-5p in tissues revealed its specificity (AUC = 1.000, 95% CI: 1.000 to 1.000, P < 0.0001), (Figure 1B).
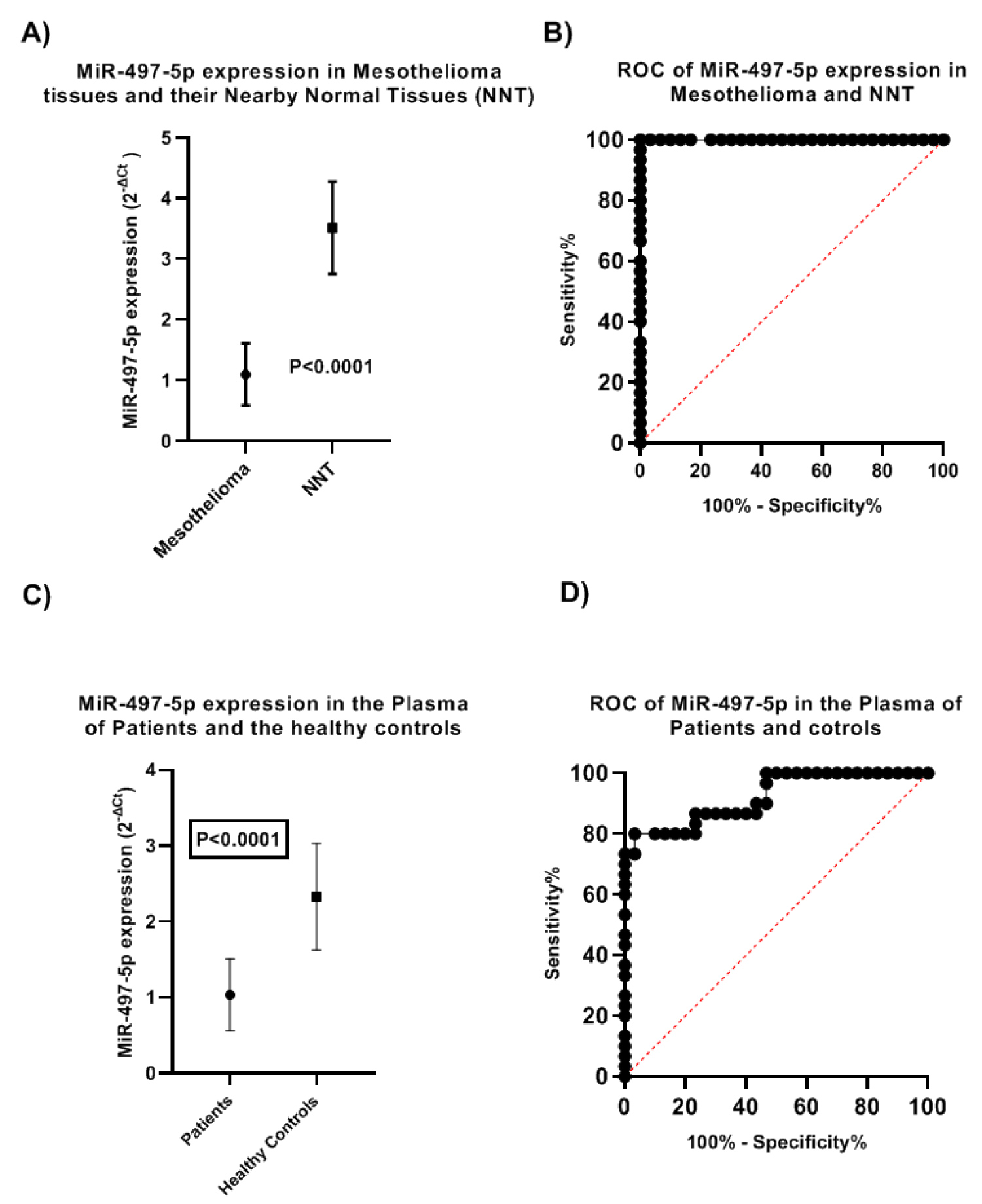 Figure 1: MiR-497-5p expression in the tissues and the plasma of mesothelioma patients and normal controls (A) Expression of MiR-497-5p in mesothelioma and their nearby normal tissues; (B) ROC curve of MiR-497-5p in Tissues; (C) Expression of MiR-497-5p in the plasma of patients and normal healthy controls and; (D) ROC curve of expression of MiR-497-5p in the plasma of patients and controls.
View Figure 1
Figure 1: MiR-497-5p expression in the tissues and the plasma of mesothelioma patients and normal controls (A) Expression of MiR-497-5p in mesothelioma and their nearby normal tissues; (B) ROC curve of MiR-497-5p in Tissues; (C) Expression of MiR-497-5p in the plasma of patients and normal healthy controls and; (D) ROC curve of expression of MiR-497-5p in the plasma of patients and controls.
View Figure 1
MiR-497-5p expression was analyzed in the plasma of patients and the healthy controls, in patients the plasma level was ranged between 0.23 and 1.72 with a mean ± SD, 1.04 ± 0.5 while in healthy controls it ranged between 1.3 and 3.7 with a mean ± SD of 2.3 ± 0.7 (Figure 1C). The difference was statistically significant (t = 8.362, P < 0.0001). ROC curve was generated to test the specificity of MiR-497-5p by qRT-PCR in plasma of patients and controls revealed (AUC = 0.921, 95% CI, 0.854 to 0.988, P < 0.0001) (Figure 1D).
The expression MiR-497-5p was compared in the plasma of patients and mesothelioma tissues. In the tissues MiR-497-5p ranged between 0.24 and 1.8 with a mean ± SD value of 1.1 ± 0.51. In the plasma it ranged between 0.23 and 1.7 with a mean ± SD value of 1.03 ± 0.47 (Figure 2), the difference was not statistically significant (t = 0.501, P = 0.618). To test the specificity of MiR-497-5p in the plasma and tissues, we calculated the ROC curve that showed (AUC = 0.561, 95% CI: 0.414 to 0.708, P 0.416) (Figure 3).
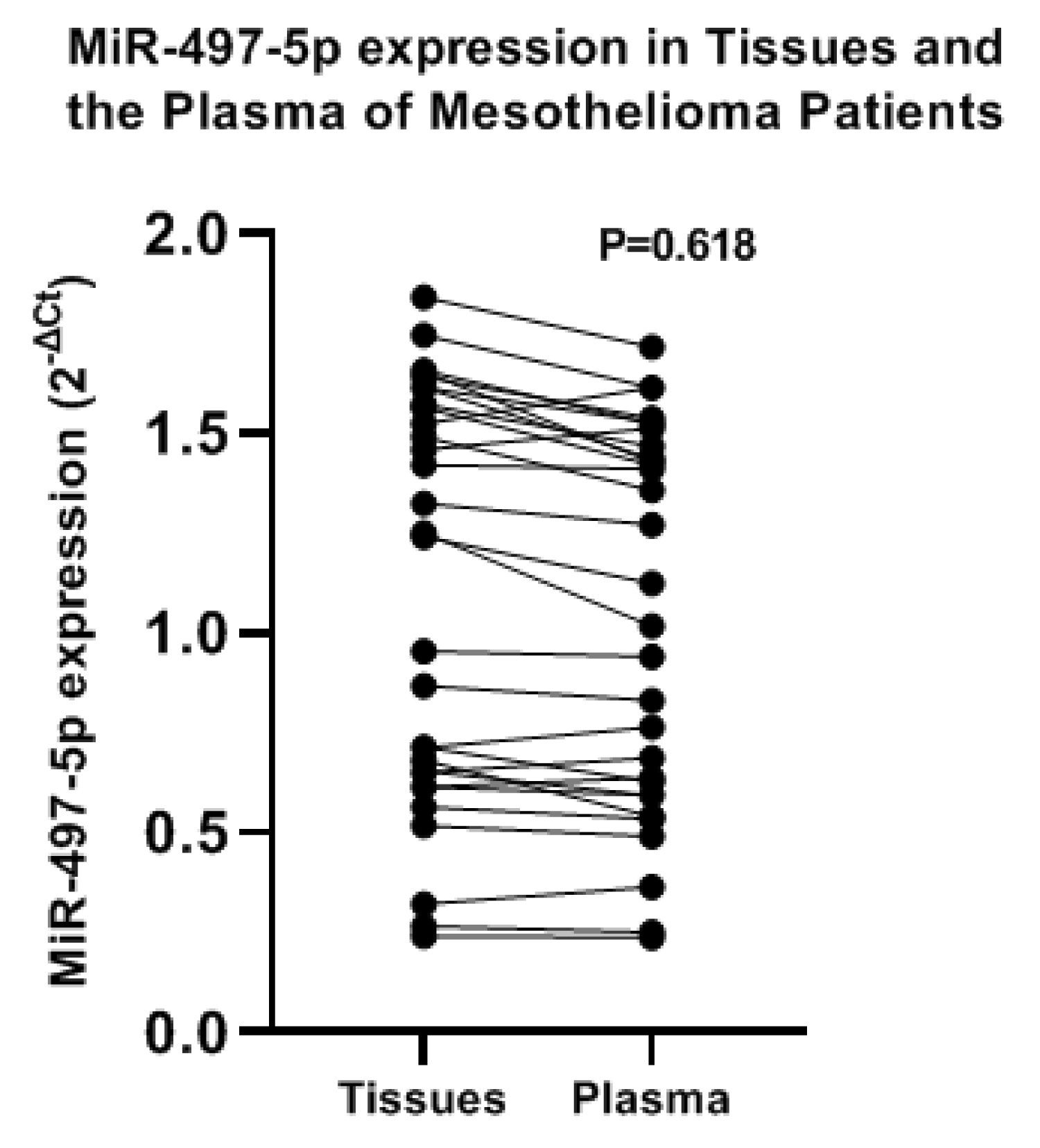 Figure 2: Expression of MiR-497-5p in the Mesothelioma tissues and the Plasma of the patients.
View Figure 2
Figure 2: Expression of MiR-497-5p in the Mesothelioma tissues and the Plasma of the patients.
View Figure 2
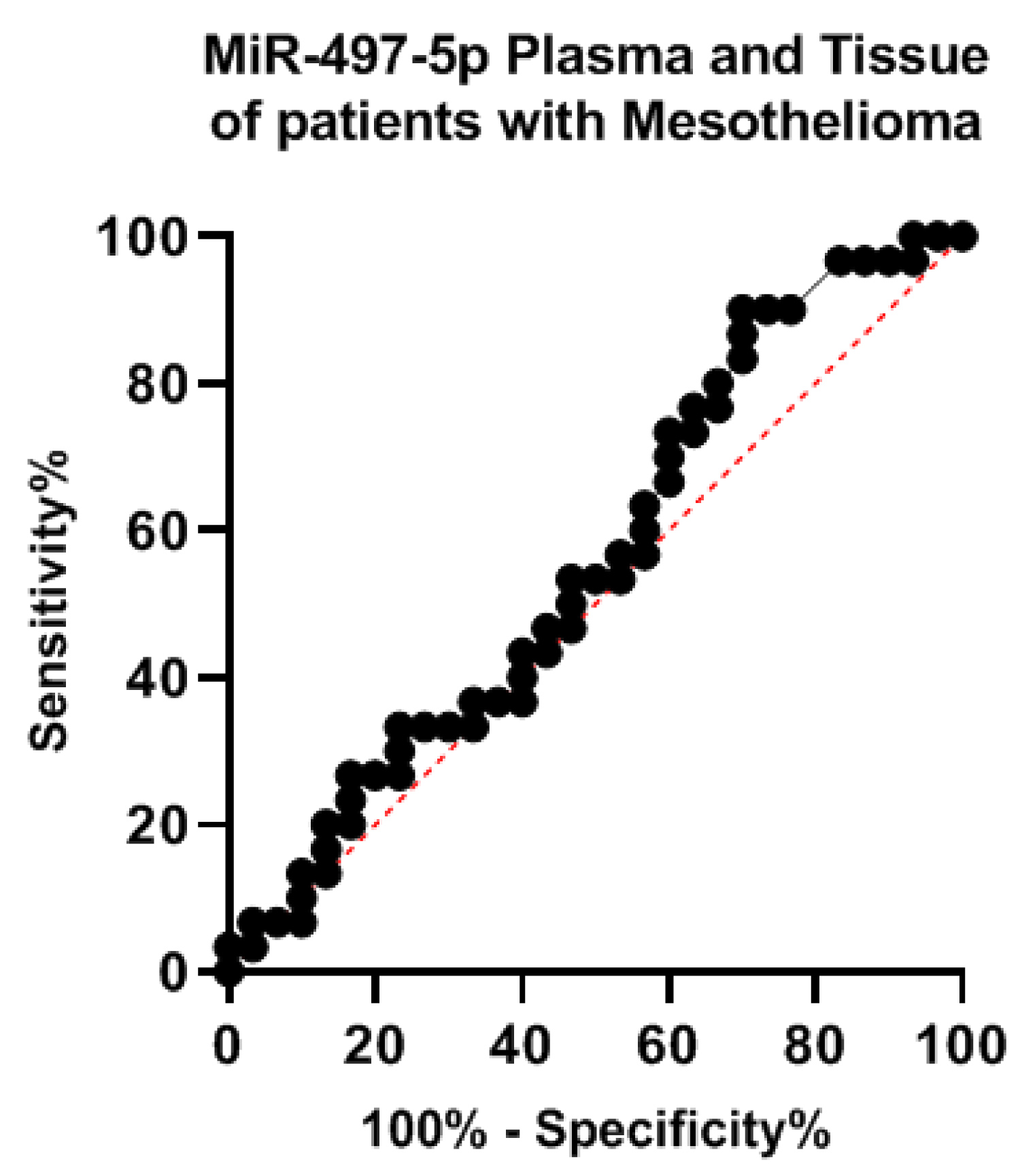 Figure 3: ROC curve for expression of MiR-497-5p in the plasma and tissues of Mesothelioma patients.
View Figure 3
Figure 3: ROC curve for expression of MiR-497-5p in the plasma and tissues of Mesothelioma patients.
View Figure 3
We evaluated the MiR-497-5p in the plasma of patients with Mesothelioma before and after surgical resection of the tumor, before surgery the mean ± SD level was 1.03 ± 0.47, after surgery the mean ± SD level was 1.4 ± 1.1, the difference was not statistically significant (t = 1.755, P = 0.086) (Figure 4).
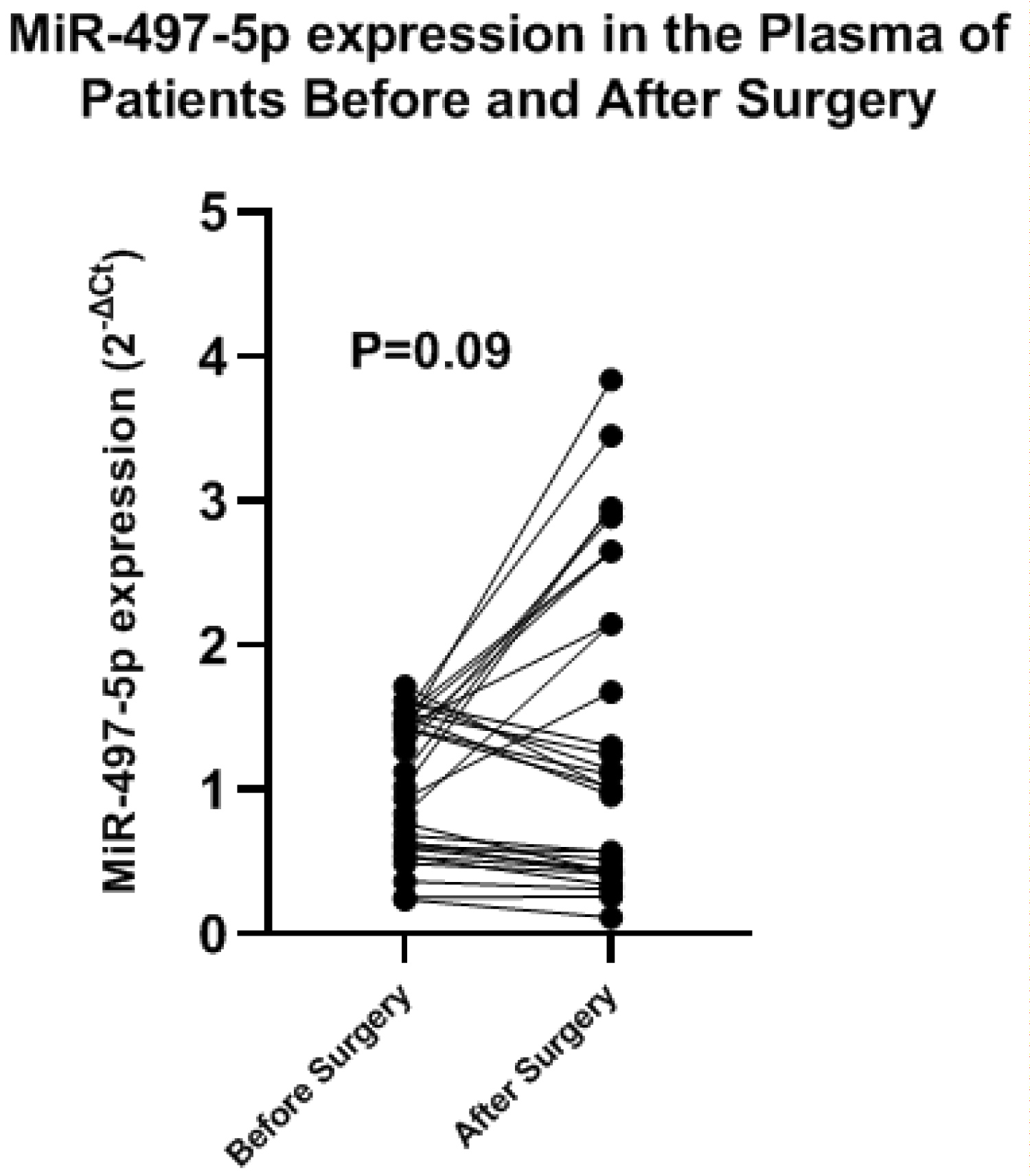 Figure 4: MiR-497-5p expressions in the plasma of patients with mesothelioma before and after surgical resection.
View Figure 4
Figure 4: MiR-497-5p expressions in the plasma of patients with mesothelioma before and after surgical resection.
View Figure 4
We tested the relation between MiR-497-5p expressions in the plasma and tissues of patients with mesothelioma and clinical prognostic factors (Table 1). We found a statistically significant correlation between MiR-497-5p expressions in the plasma and tissues and all clinical prognostic factors. Increased expression of MiR-497-5p was significantly related with female gender, good performance status of the patients, epithelioid histopathology, normal serum lactate dehydrogenase (LDH) level, normal platelets count, normal WBCs, absence of anemia, small tumor size, no lymph nodes involvement, early tumor stage, and the extent of tumor excision.
Table 1: MiR-497-5p levels in mesothelioma tissues, plasma and prognostic factors. View Table 1
Mesothelioma of the pleura is an aggressive tumor with short survival and poor prognosis. The main treatment of mesothelioma is surgery; surgical excision is only possible for early-stage tumors. To improve the outcome, patients should be diagnosed early. In the current study, we studied the role of MiR-497-5p in the plasma of patients with mesothelioma and as a non-invasive molecular marker for diagnosis and prognosis.
In the present study we MiR-497-5p in the tissues of mesothelioma and their nearby normal tissues not invaded by the tumor, MiR-497-5p was downregulated in mesothelioma tissues compared to normal tissue. In the plasma of patients, MiR-497-5p was also downregulated compared to the healthy controls. We compared the expression of MiR-497-5p in the plasma of patients and the malignant tissues excised from them; we found no significant difference between the plasma and tissue expression. In a study on patients with melanoma, MiR-497-5p was downregulated in melanoma tissues [22]. In HCC, the levels of MiR-497-5p were also lower than the normal tissues [23]. It was also reported to be downregulated in other malignancies as HNSCC [14], breast cancer [17], colorectal carcinoma [18], in non-small cell lung cancer [24], and pancreatic cancer [16].
To validate the role of MiR-497-5p as a non-invasive molecular marker, we compared the specificity in tissues and the plasma by computing the ROC curve, MiR-497-5p was specific in both tissues and plasma.
To test the role of MiR-497-5p as a potential non-invasive biomarker for follow-up of patients with mesothelioma, we measured its levels in the plasma of patients before and after surgical resection of the tumor, the plasma levels were decreased after resection but the difference was not statistically significant.
MiR-497-5p has been regarded as a sensitive biomarker with a high prognostic significance in several malignant tumors [16,25,26]. To our knowledge, the prognostic significance of MiR-497-5p in mesothelioma was not explored or tested. In the present study, we noted that downregulation of MiR-497-5p was significantly related to adverse prognostic features in mesothelioma such as male gender, poor performance status of the patient, non-epithelioid tumors, increased LDH level, high platelets count, increased WBCs, anemia, large tumors, lymph nodes involvement, incomplete tumor resection, and advanced tumor stage.
Our results revealed that circulating MiR-497-5p is a potential non-invasive molecular marker for diagnosis and prognosis of mesothelioma, but our results need to be confirmed in a study with a large number of patients.
• MiR-497-5p was downregulated in tumor tissues compared to normal tissues.
• It was also downregulated in the plasma of patients compared to the healthy controls.
• MiR-497-5p levels in the tissues and plasma were comparable.
• Patients had increased plasma levels of MiR-497-5p following surgical removal of the tumor.
• MiR-497-5p downregulation was associated with adverse prognostic features in mesothelioma.
I wish to thank professor Amal F Gharib, Professor of Medical Biochemistry and Molecular Biology, College of Applied Medical sciences, Taif University, SA.
This research did not receive any specific grant from funding agencies in the public, commercial, or not for-profit sectors.
The author declares no conflict of interests.
The data of the present study are available on request.