Erythrodermic psoriasis is a rare and life-threatening variant of psoriasis. Multiple triggering factors have been described, including emotional stress, alcohol consumption, medications, infections, less common phototherapy, photo chemotherapy, application of topical irritants, Sars-Cov 2 vaccine, HIV and hypocalcemia.
In this paper we report the first case of a patient with a flare up of erythrodermic psoriasis whose only possible trigger was uncontrolled, undiagnosed diabetes mellitus. Psoriatic arthritis was concomitantly diagnosed. He was initially managed with supportive therapy, corticosteroids, and methotrexate with a poor response.
Therefore, biological therapy with infliximab was started with a rapid clinical response and clearance of skin lesions, and resolution of joint pain.
Conclusion: Poor glycemic control may be associated with erythrodermic flares of psoriasis. Treatment should be set up based on the severity of the presentation. Timely treatment with a TNF alpha inhibitor allows rapid resolution of symptoms.
Case report, Diabetes mellitus, Erythrodermic psoriasis, Psoriatic Arthritis, Psoriasis
Erythrodermic psoriasis (EP) is an uncommon and severe variant of psoriasis. It is characterized by widespread erythema of the skin, typically involving more than 75 percent of the body surface, associated with exfoliation and skin scales. It may lead to hemodynamic and metabolic complications due to fluid loss and subsequent electrolyte imbalances, thermoregulatory disturbances due to rapid proliferation of epidermal cells and an increase of metabolic demand, infections and non-cardiogenic acute respiratory distress syndrome. This is the first case reported in the literature describing poor glycemic control as a possible trigger for an erythrodermal flare in patients with psoriasis vulgaris.
A 43-year-old male with a history of psoriasis vulgaris was presented with erythrodermic psoriasis to the emergency department of our hospital. He owned a food business, his BMI was 25 and had no other underlying health condition. He denied recent consumption of alcohol, medications oral or topic, infections, vaccines, or other compounds. He was diagnosed with psoriasis vulgaris 20 years ago, with occasional mild flare-ups, which he managed with topical corticosteroid with mild improvement.
The patient stated that 15 days ago he developed general erythema and pruritus scaling that started from his face and trunk, then spread to the rest of the body. Two weeks before the onset of these symptoms he presented polydipsia, polyuria and polyphagia, three months before he had significant weight loss, and occasionally joint pain associated with morning stiffness in the distal interphalangeal joints of both hands. He initially goes to alternative medicine who prescribes natural compounds in a magisterial formula (orotic acid, oxypurine, sulfur) with an increase in skin lesions, pain and pruritus. Upon admission, PASI score was 72.
The physical exam revealed tachycardia and mucosal dryness, generalized skin redness and severe desquamation, swelling and pain in the distal interphalangeal joints of both hands, onycholysis and onychogryphosis in the left hallux (Figure 1, Figure 2, Figure 3 and Figure 4). No signs of mucosal involvement were detected, and there was no evidence of enthesitis, dactylitis, or joint deformities. Laboratory evaluation was significant for high glucose (glucose = 410 mg/dl, normal range 80-100 mg/dL), leukocytosis and neutrophilia (WBC 19160 cells/mm3, normal range 4500-11000 cells/mm3), elevated acute phase reactants (CRP = 15 mg/dL, normal range < 2 mg/dL) and metabolic acidosis with hyperlactatemia (lactate = 2.7 mmol/L, normal range < 2 mmol/L). The diagnosis of type 2 diabetes mellitus was made, and poor chronic glycemic control was documented, given an Hba1c of 12.65%. Rheumatoid factor was negative, fulfilling CASPAR criteria for psoriatic arthritis. He was initially treated with medical support therapy (fluids, insulins), achieving proper metabolic control and hydration status. He was evaluated by the dermatology service, methotrexate 15 mg weekly and medium-dose corticosteroids were initiated, associated with topical moisturizing. The diagnosis was corroborated by skin biopsy, with findings of confluent parakeratosis and collections of hypogranular neutrophils, dilation and tortuosity of the dermal vessels with scant perivascular lymphocytic inflammatory infiltrate, compatible with psoriasis.
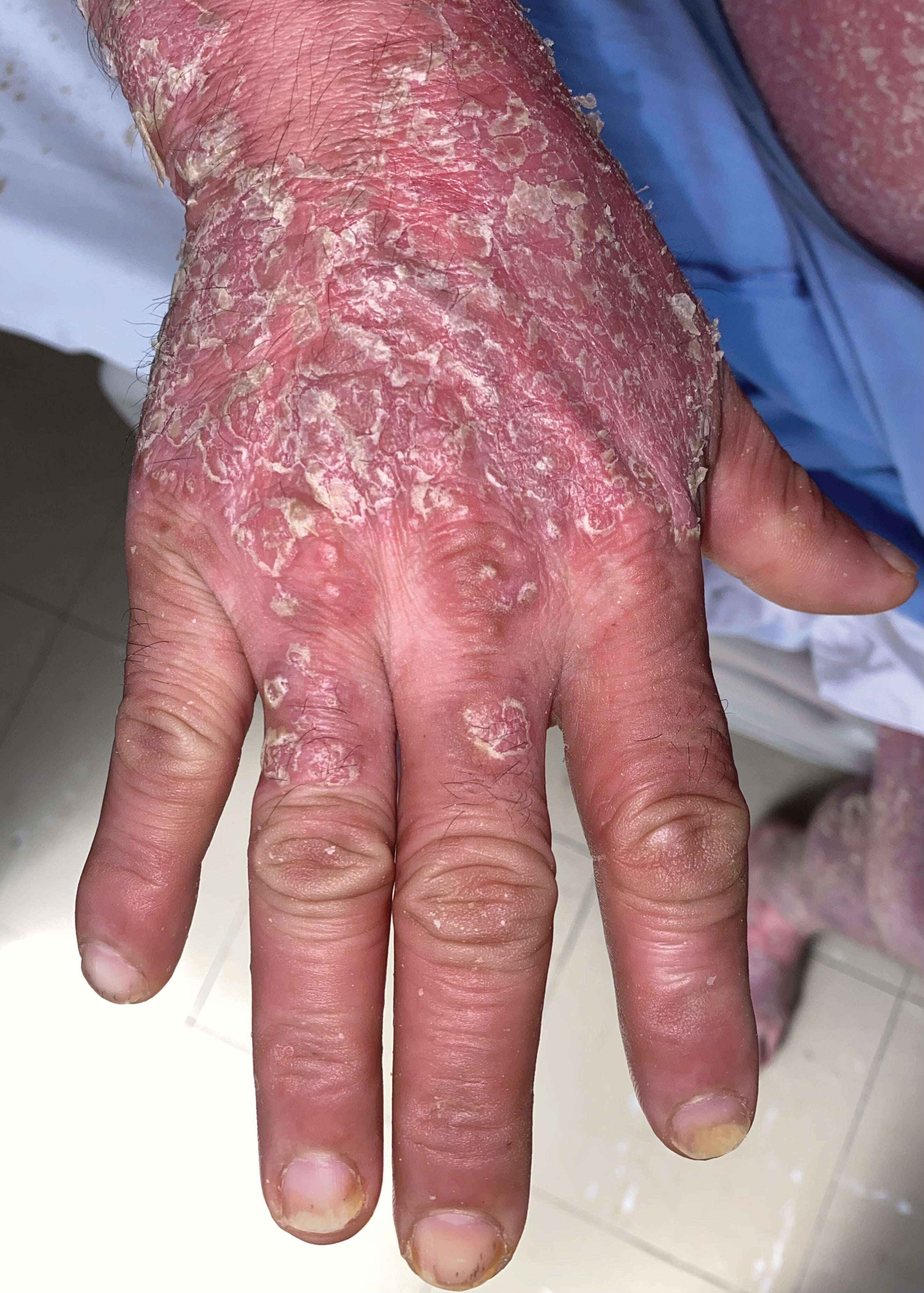 Figure 1: Erythema and severe desquamation in the face and scalp.
View Figure 1
Figure 1: Erythema and severe desquamation in the face and scalp.
View Figure 1
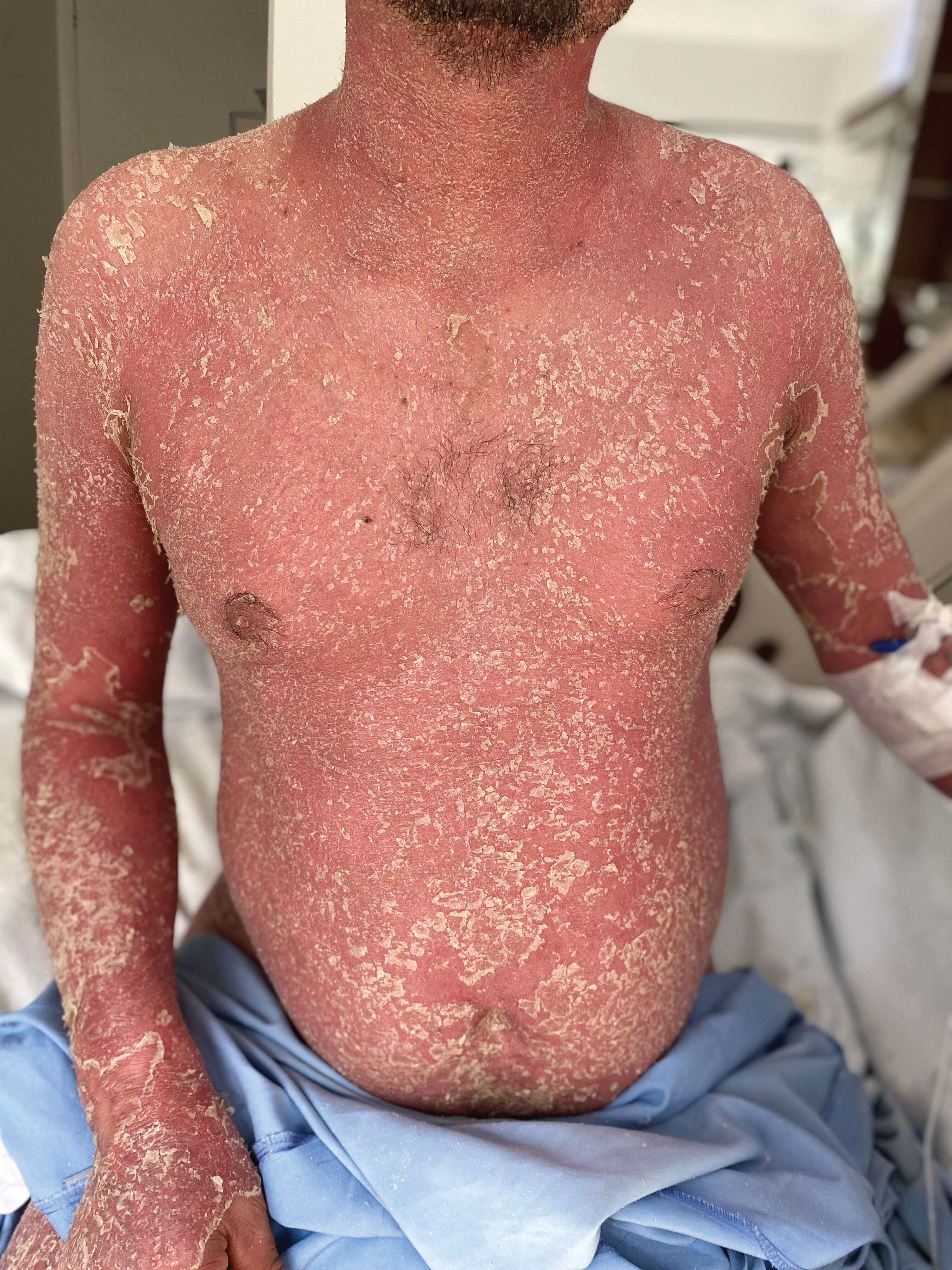 Figure 2: Erythema and severe desquamation in 90% of the total body surface.
View Figure 2
Figure 2: Erythema and severe desquamation in 90% of the total body surface.
View Figure 2
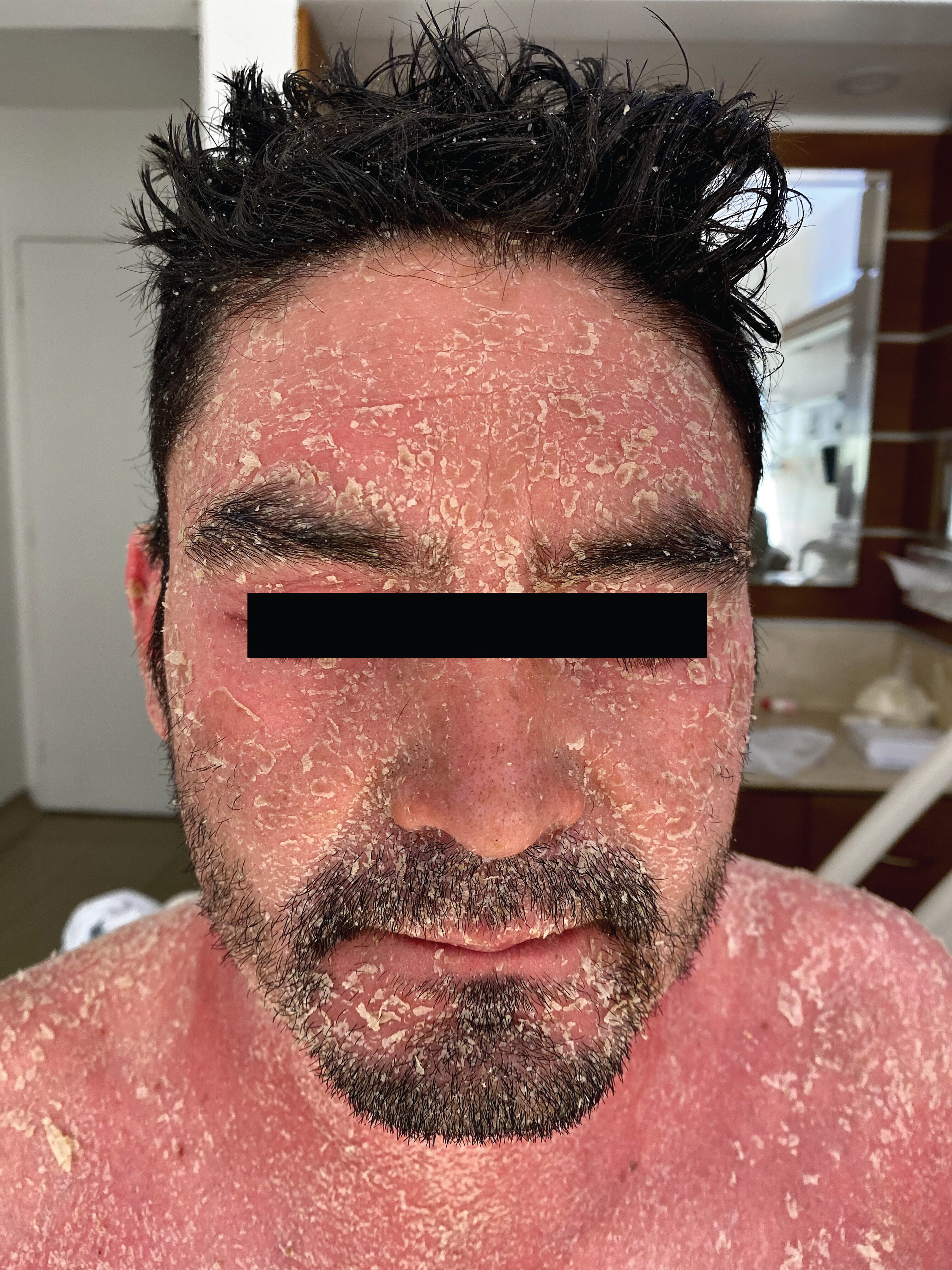 Figure 3: Infiltrated erythematous scaly plaques with thick, adherent white scales. Oil drops nails and splinter hemorrhages in the distal plate. Swelling in the distal interphalangeal joints.
View Figure 3
Figure 3: Infiltrated erythematous scaly plaques with thick, adherent white scales. Oil drops nails and splinter hemorrhages in the distal plate. Swelling in the distal interphalangeal joints.
View Figure 3
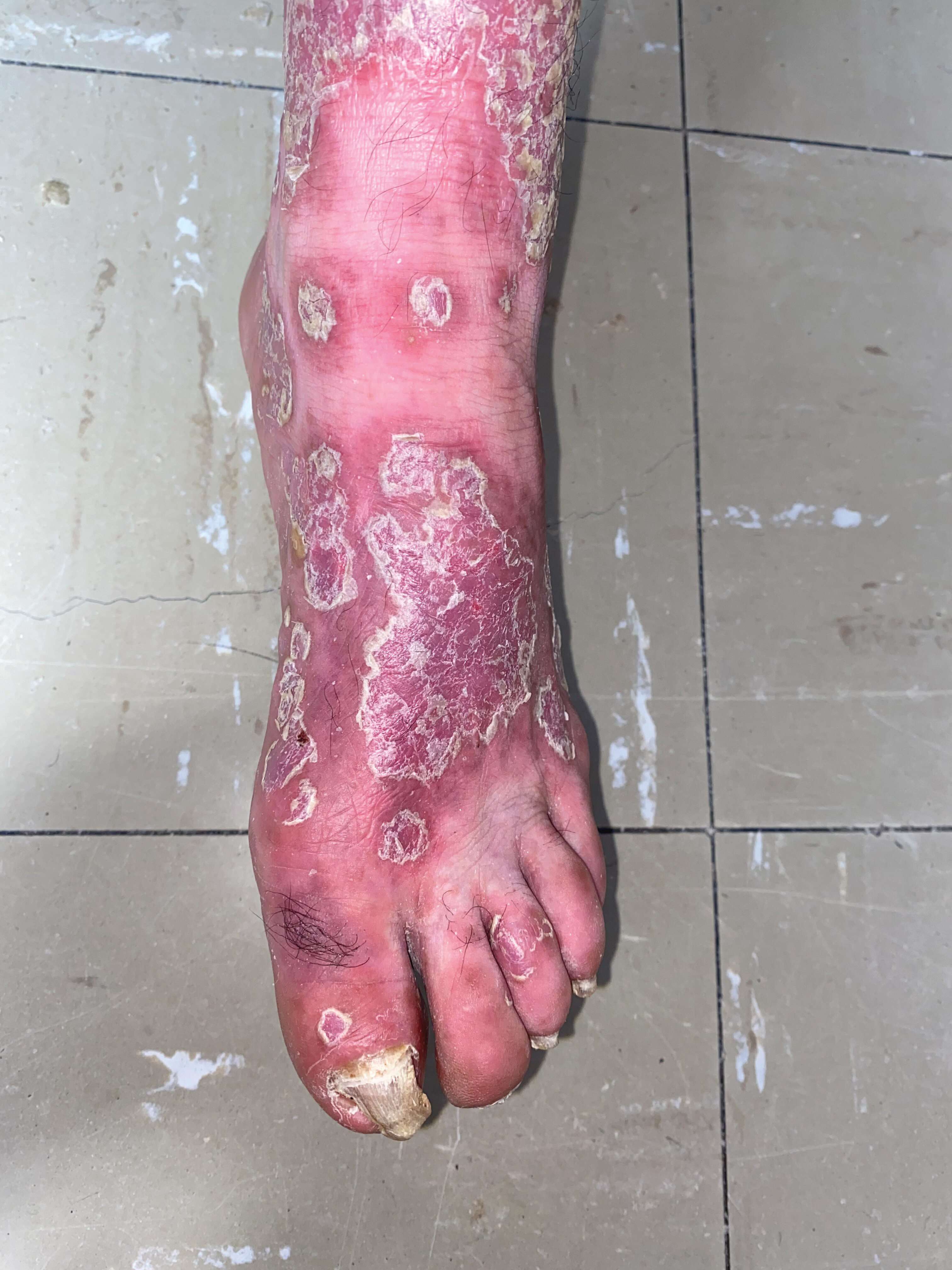 Figure 4: Infiltrated erythematous scaly plaques with thick, adherent white scales. Onychogryphosis.
View Figure 4
Figure 4: Infiltrated erythematous scaly plaques with thick, adherent white scales. Onychogryphosis.
View Figure 4
At first, the improvement of the skin lesions was little, with persistence of the erythema and scales. After 3 weeks, management was staggered and biological therapy with infliximab was started. The recovery was notable in the first week of application, with resolution of the erythrodermic component and a significant decrease in desquamation. Complications included S. aureus oxacillin-susceptible bacteremia, followed by a generalized pustular reaction in relation to the reduction in the corticosteroid dose, for which he received intravenous first-generation cephalosporin and corticosteroid titration was adjusted. He was discharged in good condition, with an improvement of 50 percent of PASI score after 5 weeks of treatment (4 weeks of methotrexate and 2 weeks of infliximab), joint pain and swelling resolved, with the indication to receive the second dose of infliximab and continue maintenance therapy with methotrexate. Until the time of this report he has not experienced any psoriasis flare-ups.
This is the first reported case of poor glycemic control as a trigger for an erythrodermic flare of psoriasis.
The worldwide prevalence of psoriasis is about 2%, but varies according to regions. It is more frequent in high income countries and in regions with older populations [1]. Erythrodermic psoriasis (EP) is the less common subtype of psoriasis, with an estimated prevalence of 1%-2.25% among psoriatic patients [2], but the one that represents the more morbidity, and may also be potentially life-threatening. Erythrodermic psoriasis may be the initial presentation of psoriasis but most patients have personal history of psoriasis.
Clinically it is characterized by the generalized development of erythema, which tends to be more rapid than gradual, and involves over 80-90% of the total body surface area, by definition more than 75 percent [3]. It is accompanied by excessive desquamation of the skin, which begins a few days after the erythema. Lesions lack sharp demarcation from uninvolved skin and lack the thickness seen in chronic plaque psoriasis. The scale tends to be finer and "powdery" [4]. The skin is itchy and very painful, predominantly in the scaly areas. Pustules are frequently identified, in a series of 50 patients with erythrodermic psoriasis, 28% were accompanied by pustules, and predominate on the lower extremities [2]. Cases have been reported, such as that of our patient, in which the pustules present in a generalized way, in these cases it is considered an overlap with the generalized pustular variant of psoriasis.
It may be associated with systemic symptoms such as tachycardia, fever, chills, malaise, myalgia, lymphadenopathy, and laboratory tests may reveal leukocytosis, eosinophilia, and anemia, as well as elevated levels of lactate dehydrogenase and ALT. As in other variants of psoriasis, it can be accompanied by psoriatic arthritis in up to 30% of patients, nail involvement, from mild pitting to total dystrophy, and palmoplantar keratoderma [2,5].
Among the possible complications, the main cause of mortality is infection, given the loss of the skin's barrier function during exfoliation. These patients are highly susceptible to infections, mainly Staphylococcus Aureus septicemia, as in the presented case [6]. Due to the exfoliation itself, there is an increase in the loss of fluids, electrolytes and proteins, which can lead to a state of shock, renal damage, hypocalcemia, hypoalbuminemia. As a result of increased cardiac output and the peripheral vasodilatation inherent in erythroderma, patients often present peripheral edema and rarely severe congestive heart failure. The edema is perpetuated by the concomitant hypoalbuminemia. There is loss of thermoregulatory function, because of the increased metabolic demands as a result of the rapid proliferation of epidermal cells. In severe cases it can be associated with non-cardiogenic respiratory distress syndrome [1].
The initial approach to therapy includes supportive management, monitoring of thermal and hemodynamic status, electrolyte replacement, nutritional support, treatment of associated infections and respiratory support if necessary. Topical treatment that improves pain and itching includes skin moisturizer, vitamin D analogs and topical corticosteroids [3,4]. Phototherapy is only encouraged when the disease becomes stable because of the risk of Khoeber phenomenon in acute phase [7].
Regarding systemic therapies, they should be started promptly. There is no high-quality scientific evidence on which to base treatment recommendations for erythrodermic psoriasis, so treatment should be dictated by the severity of disease at time of presentation and the patient's comorbidities. First-line options include cyclosporine, infliximab, acitretin and methotrexate [5]. Cyclosporine and infliximab have the advantage of a faster onset of action, however the other options are also effective. Once disease control is achieved, patients treated with infliximab or cyclosporin are often transitioned to other therapies, such as methotrexate, acitretin or other biologic therapies with an easier route of administration. The dose should be tapered to the lowest dose necessary to maintain improvement. In this case we decided to start methotrexate given the patient's socioeconomic status and joint involvement, however the initial response was insufficient.
Poulalhon, et al. conducted a retrospective trial with follow up of 28 patients receiving infliximab for severe, recalcitrant forms of psoriasis, 5 with erythrodermic psoriasis and evaluated the clinical response to treatment [8]. 3 of the 5 patients with EP reached a rapid PASI improvement of 75% or more. Another smaller serie conducted by Takahashi, et al. used infliximab to treat seven patients with erythrodermic psoriasis that had received previous systemic therapies, within 3-4 days after the first infusion all patients reported marked improvement of pruritus, skin tenderness, erythema, chills and cold intolerance, and one week after the second infusion all patients presented dramatic improvement in scaling and erythema and were released from hospital, with no adverse effects reported [9]. There are some other small series that report good outcomes with infliximab. Other TNF alpha inhibitors have been used such as adalimumab, golimumab, certolizumab and etanercept with excellent results. However, given the risk of bacteremia and sepsis in EP, it is important to note that anti-TNF agents can increase the risk of opportunistic infections and malignancy.
Methotrexate is also effective for EP but has a slower onset of action, and it is the therapy of choice in patients with peripheral psoriatic arthritis. Haustein, et al. treated 157 patients with psoriasis of which 36 were erythrodermic, with a rate of good response of 94%, similar to cyclosporine reported efficacy [10]. Response to therapy was noted within 1-4 weeks.
There is also low-quality evidence about combination therapy in erythrodermic psoriasis. Takahashi, et al. conducted an open-label, single-center study; 7 patients with erythrodermic psoriasis treated with infliximab and low doses of methotrexate with excellent outcome reported at week 6 after third infusion [9]. Heikkila, et al. also conducted a similar study with 4 patients with erythrodermic psoriasis, all with excellent outcomes reported after second or third infusion [11]. In the case of our patient, a notable improvement was seen one week after application of infliximab, however, he had previously received 3 weeks of methotrexate. Good results have also been documented with cyclosporine and etretinate, infliximab and acitretin [1].
As other available options, there are anti-IL-17 agents, an essential interleukin in the pathophysiology of psoriasis. Secukinumab and ixekizumab, two anti IL17 and brodalumab, an IL-17R antagonist, have been reported to be useful and safe for treating EP [12-14]. The use of iL-23 inhibitors has also been considered, since it is essential for the proliferation of Th17 cells, with the advantage of lower risk of infection, such as Ustekinumab. Guselkumab, a p19-directed anti-IL-23-specific biologic, used in 11 Japanese patients, demonstrated clinically significant responses at week 16 and improved quality of life through the observed 52 weeks, suggesting short- and long-term benefits [15]. There is another 52 weeks open label study in 13 patients from Taiwan with previous inadequate response to treatment, including biologics, also with good outcomes, where biologic naivety and no history of secondary failure to biologic were good factors predicting guselkumab efficacy [16]. Risankizumab also demonstrated clinically meaningful efficacy at week 16, with durable efficacy and a favorable long-term safety profile in a 180 weeks follow-up study [17].
Panitumumab, an anti-EGFR antibody, showed remarkable therapeutic effect on skin symptoms in one EP patient [18], as well as low-dose naltrexone [19]. Several case reports have shown that apremilast may be an effective option for EP patients with minor adverse events and a rapid and maintained onset of action [20,21]. In one EP patient with a high PASI score and several comorbidities refractive to previous treatments with cyclosporine, methotrexate, and adalimumab, responded to apremilast with total clearance of skin lesions after only 20 days [22].
In Boyd, et al.'s series of 50 patients with erythrodermic psoriasis, after 6 months of follow-up, 91% of patients had adequate control of their psoriasis, while 7/47 patients had at least one subsequent episode of erythroderma. Mortality rates attributable to disease are variable between 9 and 64 percent [2].
Possible triggers include medication exposure, such as systemic glucocorticoids [2], withdrawal of systemic antipsoriatic medications such as methotrexate and cyclosporine [1], oral and topical retinoids, trimethoprim-sulfamethoxazole [23], bupropion [24], pegylated interferon-alpha and ribavirin [25]. Other factors that have been reported to trigger erythrodermic psoriasis include emotional stress, alcohol consumption [2], Sars-Cov2 vaccines [26], phototherapy or photochemotherapy [2], application of topical irritants and hypocalcemia. HIV infection is a risk factor for EP, and is correlated with the level of immunosuppression [27]. In our patient, all these factors were absent, and poor metabolic control was documented as the only possible triggering factor.
Due to its inflammatory nature, it has been postulated that psoriasis is a systemic entity, more than one affecting only the skin. The pathogenesis of psoriasis is a complex, immune-mediated process that results in damage beyond the skin. The inflammatory pathway of TNF-α-IL-23-Th17 predominates, inducing chronic inflammation, uncontrolled keratinocyte proliferation, dysfunctional differentiation of keratinocytes, and neovascularization [1]. Because of chronic inflammation, patients with psoriasis have increased risk of hyperlipidemia, hypertension, coronary artery disease, metabolic syndrome, obesity, mood disorders, and about one third of psoriasis patients have joint and tendon involvement [28]. Metha, et al. evaluated systemic inflammation vía [18F]-fluorodeoxyglucose positron emission tomography-computed tomography (FDG-PET/CT) in six patients with moderate to severe psoriasis, and revealed subclinical inflammation in the blood vessels, predominantly the aorta, joints, and liver [29].
Diabetes mellitus (DM) is a disease characterized by a chronic hyperglycemic state with an associated spectrum of well known complications, such as nephropathy, retinopathy, neuropathy, cardiovascular disease, some cancers, dementia, non alcoholic fatty liver disease among others. Psoriasis and Diabetes Mellitus share many comorbidities, and have been associated with each other. It has been postulated that rather than being a risk factor, both pathologies share pathophysiological mechanisms that relate them to each other, including genetic and epigenetic changes, inflammatory pathways, environmental factors and insulin resistance leading to organ damage [30].
Psoriasis patients have shown to have higher fasting plasma insulin than healthy controls, and patients with diabetes and psoriasis exhibit worse metabolic measures compared to diabetic patients without psoriasis (fasting plasma glucose, HbA1c, HOMA-IR, triglyceride and LDL-C levels, plasminogen activator inhibitor-1, soluble adhesion molecules, matrix metalloproteinase, and adipocytokines) [31].
Regarding genetic changes, Wang, et al. identified 89 DM susceptibility loci in 4500 psoriasis patients in China, and identified 4 genes that were significantly shared between DM and psoriasis. PTPN22, JAZF1 and ST6GAL1 genes have been found to be disturbed in both entities, compromising signaling pathway of a T cell receptor, beta cell function and antigenic differentiation necessary for T-cell activation respectively [32]. A polymorphism of the CDKAL1 gene has also been involved in the production of insulin, which is decreased in keratinocytes and immune cells in patients with psoriasis [33]. At last, it has also been postulated that the miR-21-PDCD4 axis plays a key role in DM and in psoriasis, since it has been seen to be increased in helper T cells in patients with psoriasis, and has been implicated in type 1 DM promoting genes [34].
On the other hand, chronic inflammation is a common denominator both in psoriasis and DM, as well as in the comorbidities with which they are related. It has been described that chronic elevation of TNFalpha secreted both in the dermis and in adipose tissue is associated with disturbance of adiponectin transcription, and increases the expression of pro-inflammatory leptin and anti-inflammatory adiponectin [35]. At the metabolic level, a study by Gyldenlove, et al. described a decreased incretin effect (39% vs. 57%, p = 0.02) and gastrointestinal-mediated glucose disposal in non-diabetic patients with moderate-to-severe psoriasis compared to healthy controls matched for age, gender and BMI, which under normal conditions plays an essential role in the prevention of beta cell apoptosis [36]. Finally, psoriasis has been associated with the development of insulin resistance, by mechanisms other than chronic hyperglycemia, more related with epigenetic changes associated with chronic inflammation [35].
This becomes relevant mainly when talking about therapeutics. Some therapies may be potentially used to treat both psoriasis and DM. Non-pharmacologic interventions such as lifestyle modification have been proved to be beneficial in both patients with DM and psoriasis [30,31]. Some medications such as analogs GLP-1 have been described to improve severity of psoriasis in diabetic patients, as well as metformin that has been associated to reduce risk of psoriasis [37]. Thiazolidinediones have also been associated with clinical improvement in patients with chronic plaque psoriasis through a systematic review and meta-analysis [38].
Therefore it is clear that psoriasis is related to diabetes, and our patient had a higher risk of developing this pathology given his history of vulgar psoriasis. However, the key point of this case is to explain what conditioned the patient to develop erythroderma, and poor metabolic control was the only factor identified that in this case could induce a condition of systemic stress, and then could predispose the development of erythroderma. The association between diabetes and the erythrodermic variant of psoriasis has not been described in the literature, but it surely implies this wide range of disruption in the inflammatory and metabolic pathways in both entities. More research is needed to evaluate the mechanisms implicated in this variant of psoriasis.
As strengths and limitations in our approach to this case, we decided to use methotrexate as the first line therapy given his socioeconomic status, with coverage for the arthritic component of psoriasis. However, understanding the delayed onset of drug action the initial response was insufficient, so we recommend starting targeted therapy early in severe cases. Depending on the context and comorbidities, another type of immunosuppressant such as methotrexate could be started concomitantly to treat both the acute and late stage of the disease, already having established a long-term concomitant therapy to make the transition afterwards, and thus avoid new flare-ups.
In conclusion, diabetes mellitus and psoriasis are two different but similar entities that share not only some pathophysiological mechanisms but also multiple comorbidities. Chronic hyperglycemia could be a triggering factor for erythrodermic psoriasis. Treatment should be set up based on the severity of the presentation. Interferon alpha inhibitors are recommended as first line to treat EP, however as the evidence improves with other biologics, they may be likely to be established as good options with a better safety profile. More research is needed to evaluate the mechanisms implicated in this variant of psoriasis and diabetes.
Consent was given by the patient for the publication of the photographs and medical information, with the understanding that this information may be publicly available.