To evaluate the 25-OH vitamin D status in Rheumatoid Arthritis (RA) patients and to assess the relationship between serum 25-OH vitamin D levels with disease activity and anti-cyclic citrullinated peptide antibody levels.
In our study, 42 RA patients were selected according to the ACR Criteria and 50 healthy participants were recruited for comparison, as controls. After a detailed medical history and anthropometric evaluation, all participants were subjected to CRP analysis and their Disease Activity Scores (DAS28-CRP) were calculated using DAS calculator. Serum 25-OH vitamin D levels and anti-CCP antibody were measured by commercial ELISA kits.
We found that the mean 25-OH vitamin D levels of the patients with RA were not different than the controls (64.03 ± 41.36 vs. 58.90 ± 24.27). 45.24% of RA patients had 25-OH vitamin D deficiency as compared to 43.48% of controls. This level was statistically significant between the 25-OH vitamin D deficient RA patients and the 25-OH vitamin D deficient controls (p = 0.013). Our study population consisted of mostly female arthritic patients (about 88%). The mean age of RA patients was 44.62 ± 13.47 years and the mean disease duration was 53.6 months; mean DAS28CRP was 4.9 ± 0.97. The mean age of the healthy controls were 38.55 ± 13.27 years and 38% were female participants. In RA patients, a negative correlation was observed between serum 25-OH vitamin D level and the DAS-28 CRP (r = -0.236, p = 0.038). The anti-CCP antibody levels in patients with disease duration of less than one year is inversely correlated with 25-OH vitamin D (r = -0.77, p = 0.027). 25-OH vitamin D deficiency is reported in elderly people from different parts of the world. The deficiency is also frequent in the younger population.
We observed 25-OH vitamin D deficiency in our RA population and it correlates inversely with the disease activity score in RA patients and anti-CCP antibody levels in patients with disease duration of less than one year.
Anti-CCP antibody, Disease activity, Disease duration, Rheumatoid, 25-OH vitamin D
Rheumatoid Arthritis (RA) is an autoimmune inflammatory disease characterized by polyarticular inflammation of the synovial tissue [1]. It affects approximately 1% of the population, with a female to male ratio of 3:1. The 2011 world report on disability found that nearly 11.9 million people worldwide have disability caused by RA [2]. Worldwide low vitamin D concentrations are prevalent for all age groups; however, the older population is especially at high risk for vitamin D deficiency due to the decreased cutaneous synthesis and dietary intake of vitamin D. Several studies have revealed a high prevalence of vitamin D deficiency even in areas of the world that receive ample of sunlight [3].
The prevalence of rheumatoid arthritis in Indian population has been estimated to vary between 0.3% - 0.75% and based upon 2011 census; more than 36 million patients are expected to be suffering from rheumatoid arthritis in India [4]. The prevalence of RA in India is similar to that reported in the developed countries. It is higher than that reported from China, Indonesia, Philippines and rural Africa. These findings are in keeping with the fact that the north Indian population is genetically closer to the Caucasians than to other ethnic groups. The prevalence of hypovitaminosis D (< 20 ng/mL) among young adults in India, is around 70% with a slightly higher preponderance in women (76%). The inadequate sun exposure is an important cause of the hypovitaminosis D [5]. Deficiency of 25-OH vitamin D in India is very common (50-90%) in all age groups and both sexes. More than 90% individuals above 50 years have 25-OH vitamin D deficiency in India [6].
The early diagnosis of RA is essential, as the progression occurs within 2 years of disease onset and if not aggressively treated early, joint destruction is irreversible. RA patients are often difficult to diagnose early, because they do not always show typical symptoms and signs and they may not fulfil the ACR (American College of Rheumatology) classification criteria. RF (Rheumatoid Factor) assay is 54% to 88% sensitive and 48% to 92% specific, and it is frequently detected in hepatitis and other connective tissue diseases. Among the new antibodies described in the recent years in patients with RA, Anticyclic Citrullinated Peptide (Anti-CCP) antibodies are very specific for RA [7]. A greater number of RA studies in India may include the anti-CCP antibody assay in the future, as its ability to diagnose patients with RA correctly, is established. In a study conducted in India, to investigate the sensitivity and specificity of anti-CCP antibodies in RA patients with respect to patients with non-RA rheumatic diseases, the sensitivity of the assay was 85% and the specificity was 90%. Anti-CCP antibodies are useful in the detection of early arthritis [8]. Moreover, anti-CCP is detected in less than 1% of healthy individuals; it can even appear before RA is clinically evident and detectable [9].
Emerging evidence suggests that 25-OH vitamin D plays an important role in immune regulation [10]. Preliminary studies suggest that low levels of 25-OH vitamin D may be common in Rheumatoid Arthritis (RA) [11] and is involved in the pathogenesis of RA as well as the activity of RA [2]. Vitamin D receptors are found on several immune cells and in vitro studies have shown that vitamin D metabolites modulate T cell proliferation and dendritic cell function. Vitamin D is a secosteroid hormone involved in bone and calcium metabolism. It is involved in the regulation of calcium homeostasis, as it regulates calcium absorption from the gastrointestinal system. The hormone is synthesized in the skin by the action of ultraviolet irradiation [12]. It is produced in the skin from 7-dehydrocholesterol under the influence of sunlight as well as intake from the diet. In the liver, vitamin D is converted to 25-hydroxyvitamin D, which is the specific vitamin D metabolite that is measured in serum to determine a person's vitamin D status [13]. In the kidneys and extrarenal tissues, 25-OH vitamin D is converted into calcitriol, the biologically active form of vitamin D, which mediates its biological effects by binding to the vitamin D receptor. Both genetic and nongenetic (e.g. environmental, infectious, hormonal) elements may be responsible for the prevalence of RA. 25-OH vitamin D might be one of the environmental factors relevant with RA. There is a higher prevalence of osteoporosis in RA patients, and clinicians often supplement vitamin D together with calcium for this reason.
Recent evidence demonstrates that vitamin D may correlate inversely with occurrence, development, disease activity and flare ups of RA [14-16]. There have been conflicting results regarding the correlation between the disease activity levels in RA patients and blood levels of 25-OH vitamin D and 1,25(OH)2D. The present study was undertaken to estimate the prevalence and determinants of 25-OH vitamin D deficiency in patients with RA, and to analyse the correlation of 25-OH vitamin D with disease activity and anti-cyclic citrullinated peptide antibody.
In our study, 42 patients with RA, diagnosed by American College of Rheumatology (ACR) criteria [17] and after radiological analysis, were included. Recruitment of the patients was from OPD of Sir H. N. Reliance Foundation Hospital and Research Centre. The control group of 50 healthy participants were enrolled of which 38% were female participants. The mean age of the healthy controls were 38.55 ± 13.27 years. The patients below 18 years of age, pregnant patients, patients with positive serology for HIV, hospitalized patients and patients with history of infection in last one year were excluded from the study.
All patients were evaluated for their systematic involvement. Besides this, at the time of recruitment, the physical findings such as height, weight, blood pressure, duration of the disease and DAS28-CRP score and CRP analysis was done. Patient Global Assessment (PGA) of disease activity, swelling, morning stiffness were noted. The DAS Score was calculated by counting number of swollen joints (out of 28) and the tender joints (out of 28) [17]. The treatment for RA patients was recorded which included Disease Modifying Anti-Rheumatic Drugs (DMARDs) with calcium and vitamin D supplements.
10 ml of blood was collected through peripheral venipuncture from all the study participants. The serum was separated and stored at -80 ℃ for determination of 25-OH vitamin D (Immunodiagnostic Systems Ltd, USA; Catalogue No: AC-57F1) and anti-CCP antibody (Eurodiagnostica Germany; Catologue No: RA-96 PLUS). The specifications of 25-OH vitamin D Elisa kit is: LOD: 25 nmol/L; range: 25-250 nmol/L; mean linearity = 102%; mean recovery = 101%; specificity = 75-100%. The specifications of anti-CCP antibody Elisa kit is: LOD: 25 U/mL; range: 25-3200 U/mL; mean recovery = 101%; specificity = 97.5%. CRP analysis was detected by agglutination method on fully automated XL-300 in a diagnostic laboratory. Calculation and evaluation of disease activity using DAS28-CRP according to the formula on the DAS website is as given below:
DAS28-CRP = 0.56*sqrt(TJC28) + 0.28*sqrt(SJC28) + 0.36*Ln(CRP+1) + 0.014*GH+0.96 [18].
(TJC: Tender Joint Count; SJC: Swollen Joint Count; CRP: C-reactive Protein; GH: General Health on a 100 mm Visual Analogue Scale. The disease activity is considered 'high' with the score of > 5.1, 'moderate' is defined as score 3.2-5.1, 'low' with < 3.2, and the score of < 2.6 for diseases in 'remission'. With regard to age, the patients were divided into 3 groups: (1) Younger than 30 years; (2) 30 to 50 years and (3) Older than 50 years.
The analyses were performed using the Statistical Package for Social Sciences SPSS) software, version 21.0 (SPSS, Chicago, IL, USA). The numerical data confirmed to a normal distribution was assessed by Kolmogorov-Smirnov test. Independent t-test was used to evaluate the differences between patients and controls. One-way ANOVA was used to compare the sub-groups in RA group. Scatter plots with linear regression line were drawn. To determine the correlation between the disease activity and 25-OH vitamin D status in RA patients Spearman's correlation coefficient was used. We used the threshold of 50 nmol/l to define 25-OH vitamin D deficiency based on recent literature [19]. To evaluate distribution of patients and controls across 25-OH vitamin D status groups (deficiency, insufficiency and sufficiency), Chi-square test was applied. Logistic regression was used to estimate the Odds Ratio (OR) for calculating the 25-OH vitamin D deficiency in RA patients and the controls. The significance cut-off value (P) was fixed to 0.05.
Table 1 represents the characteristics of RA patients and controls. Our study population consisted of mostly female arthritic patients (88%) with mean age of 44.62 ± 13.47 years and the mean disease duration was 53.6 months; mean DAS28CRP was 4.9 ± 0.97. The mean SJC and TJC counts in the RA patients were 5.86 ± 3.85 and 9.05 ± 4.38 respectively. The mean age of the healthy controls were 38.55 ± 13.27 years and 38% were female participants. The CRP levels (21.32 ± 34.04 vs. 7.46 ± 9.16) mg/l in RA patients was significantly increased in patients as compared to the controls indicating inflammation in the patients of our study. Around 42.1% of patients were being treated with Methotrexate, 15.8% were being treated with Leflunomide, 13.2% were being treated with Sulphasalazine while 65.8% patients were being treated with Hydroxychloroquine. All the patients were taking vitamin D supplements. None of the patients were given glucocorticoids. We found that the mean of the 25-OH vitamin D levels of the patients with RA was not different than the controls (64.03 ± 41.36 vs. 58.90 ± 24.27) nmol/l and not statistically significant (p = 0.537). Also, the anti-CCP antibody levels were observed to be significantly higher in RA patients (368.63 ± 284.52 U/ml) as compared to the controls (21.9 ± 3.24 U/ml). Around 80% of the RA patients were anti-CCP antibody positive. There is a negative correlation between anti-CCP antibody levels and 25-OH vitamin D in RA patients (n = 17) with disease duration of less than one year (r = -0.77, p = 0.027)
Table 1: Characteristics of rheumatoid arthritis patients (N = 42) and Controls (N = 50). View Table 1
In Table 2, RA patients were classified in groups of 25-OH vitamin D deficiency (< 50 nmol/l), insufficiency (50-75 nmol/l) and sufficiency (> 75 nmol/l) as groups done by Attar SM, et al. [20]. As observed, 25-OH vitamin D deficiency was more prevalent in RA group compared to the Control group. As seen from the table, prevalence of serum 25-OH vitamin D deficiency in RA and controls was 45.24% and 43.48%, respectively. This level was statistically significant between the 25-OH vitamin D deficient RA patients and the 25-OH vitamin D deficient controls using the independent t-test (p = 0.013). In logistic regression analysis, to evaluate the 25-OH vitamin D deficiency in RA patients and controls, the odds of 25-OH vitamin D deficiency in RA patients was 1.1 times higher than 25-OH vitamin D deficient controls (OR = 1.10, 95% CI, 0.995 - 1.221, P = 0.06). Also, 16.67% of RA patients were 25-OH vitamin D insufficient whereas 26.1% of the controls had 25-OH vitamin D insufficiency.
Table 2: Comparison of serum levels of 25-OH Vitamin D sufficiency in RA patients and Controls using the independent t-test. View Table 2
Table 3 represents the clinical parameters of the RA patients according to the Disease Activity Score (DAS28-CRP). Around 69.05% of the RA patients have 'high' disease activity score while 30.95% have moderate Disease Activity Score (DAS28-CRP). The mean 25-OH vitamin D level in high disease activity group (n = 29) is 58.52 ± 55.13 nmol/l which is significantly low as compared to that in moderate disease activity group patients (96.60 ± 44.13) nmol/l.
Table 3: Clinical features in RA patients between High DAS28-CRP and moderate DAS28-CRP. View Table 3
Figure 1 shows that the correlation between serum 25(OH) D level and the Disease Activity Score-CRP (DAS-28 CRP) in RA patients. There is a negative correlation between the serum 25-OH vitamin D level and the DAS-28 score (r = -0.236, p = 0.038).
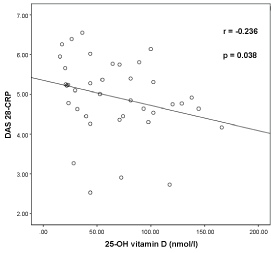 Figure 1: Graphical representation of relationship of 25-OH Vitamin D and Disease Activity Score (DAS28-CRP) in Rheumatoid Arthritis Patients (n = 42). View Figure 1
Figure 1: Graphical representation of relationship of 25-OH Vitamin D and Disease Activity Score (DAS28-CRP) in Rheumatoid Arthritis Patients (n = 42). View Figure 1
Figure 2 represents the histogram showing the range of 25-OH vitamin D levels as seen in different age groups in RA patients in our study. All patients were divided into 3 groups: (1) Younger than 30 years; (2) 30 to 50 years and (3) Older than 50 years. Studies show that younger patients have lower serum 25-OH vitamin D levels than older patients with RA. It is observed that younger age is associated with lower serum 25-OH vitamin D levels [21]. In our study, the 25-OH vitamin D levels in the groups of RAs as regards to distribution of age were not statistically different.
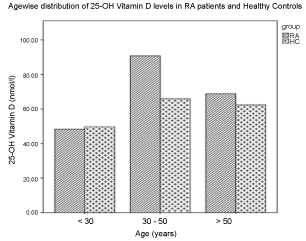 Figure 2: Histogram representing the levels of 25-OH vitamin D with respect to age-wise distribution in RA patients and healthy controls. View Figure 2
Figure 2: Histogram representing the levels of 25-OH vitamin D with respect to age-wise distribution in RA patients and healthy controls. View Figure 2
25-OH vitamin D estimation was used in our study to determine the status of vitamin D levels because serum 25-OH vitamin D with a half-life of 2-3 weeks was the best indicator of reflecting the overall vitamin D status, compared to the half-life of 1,25-OH vitamin D which is only 3 to 4 hours [22]. The patients with very active disease (DAS score > 2.1) are at higher risk of 25-OH vitamin D deficiency.
Our study showed a significant inverse correlation between the level of serum 25-OH vitamin D and DAS28-CRP score. Epidemiological studies suggest that adequate vitamin D levels decrease the risk of developing autoimmune diseases such as multiple sclerosis, inflammatory bowel disease, RA, and type I diabetes mellitus [23,24]. Several studies have evaluated the correlation between 25-OH vitamin D levels and RA activity. In Abourazzak's study [25], serum 25-OH vitamin D concentration was inversely associated with DAS. In a study of 158 RA patients, Moghimi, et al. [26], found an inverse relationship between DAS and 25-OH vitamin D levels. Also, Zakeri, et al. [27] and Chen, et al. [28] showed that 25-OH vitamin D deficiency is linked with the activity levels in RA. The study by Patel, et al. also observed a strong inverse correlation between serum 25-OH vitamin D level and DAS score at disease onset only, but not in patients with disease duration longer than 1-2 years [29].
Our results are in accordance with the findings by Turhanoglu, et al. [24]. The small number of patients included in our study may be seen as a limited sample. So, other studies with a larger patient number should also be considered. Our findings are in concordance with the findings by Kostoglou-Athanassiou, et al. [12], Rossini, et al. [10], Haque and Bartlett [11]. Also, Welsh, et al. [30], Cutolo, et al. [31] and Kerr and colleagues [32] stated that 25-OH vitamin D deficiency is linked with increased disease activity in RA. However, other studies did not find a significant correlation between 25-OH vitamin D and RA activity scores [16,33,34]. In the study by Braun-Moscovici & colleagues [16] they found no correlation between vitamin D levels and disease activity among 85 patients with Rheumatoid Arthritis. However, overall their subjects had high disease activity and low 25(OH) D3 levels, accounting for a high vitamin D deficiency rate, which might have influenced the study outcome and the lack of correlation between disease activity. Baker, et al. [34] have shown that serum levels of 25-OH vitamin D did not correlate with RA activity and response to treatment was similar in patients with different levels of vitamin D. Studies conducted by Song and colleagues showed a correlation between vitamin D intake and the risk of RA [35]. Many Indian studies have reported low 25-OH vitamin D levels in the general population, in spite of abundant sunshine and exposure [5].
We also observed a negative correlation between anti-CCP antibody levels and 25-OH vitamin D in RA patients (n = 17) with disease duration of less than one year (r = -0.77, p = 0.027). The correlation between anti-CCP antibody and DAS score was studied by Wang Yanan, et al. According to Wang Yanan, et al. there was an inverse correlation between anti-CCP antibody and disease activity in their study participants. So, anti-CCP antibody level can be used as an indicator for supplementation treatment of vitamin D in the RA patients [2]. Serum 25-OH vitamin D level was negatively correlated to anti-CCP antibody level and disease activity, which implied the therapeutic role of serum 25-OH vitamin D in RA. In the study conducted by Sahebari, et al. anti-CCP levels showed a negative correlation with serum 25-OH vitamin D in new cases [36].
Vitamin D has modulatory effects on B lymphocytes and Ig production. Many immune cells containing vitamin D receptors (like monocytes, macrophages, dendritic cells, and activated T and B cells) possess the enzymatic machinery to convert vitamin D into its active form [37].
B lymphocytes play several critical roles in the pathogenesis of rheumatoid arthritis. They are the source of the rheumatoid factors and anticitrullinated protein antibodies, which contribute to immune complex formation and complement activation in the joints [38]. B cells can produce cytokines, chemokines, adhesion molecules, and angiogenesis factors that can potentially play important roles in the pathogenesis of RA. The most prominently studied aspect of B cell biology in RA is the production of antibodies against exogenous and self-antigens (such as the Fc region of IgG, the target of rheumatoid factor), and cyclic citrullinated peptides. Discovery of serum autoantibodies was one of the earliest indications of a role for B cells in RA [39]. The two most widely studied autoantibody systems included in the clinical management of patients with RA, are RFs and Anticitrullinated Protein Antibodies (ACPA) [40].
It is also reported that calcitrol inhibits plasma-cell differentiation and B-cell proliferation [41]. In RA disease, it is believed that the antigen-dependent T cell triggers an immune response essentially of the Th1 type. This activation has multiple effects, including activation and proliferation of endothelial and synovial cells, recruitment and activation of proinflammatory cells, secretion of cytokines and proteases by macrophages and fibroblast-like synovial cells, and production of auto-antibodies.
The mean 25-OH vitamin D levels were found to be similar to RA patients when compared to controls in our study. Attar, et al. [20] also observed that the mean 25-OH vitamin D levels in patients with RA were similar to the Control group (32.3 ± 14.4 nmol/l) vs. (31.4 ± 16.4 nmol/l). Turhanoglu, et al. also reported that the 25-OH vitamin D levels in RA patients and controls were similar [24]. Several studies have confirmed that 25-OH vitamin D deficiency is common in RA. Our study showed that there was a higher frequency of 25-OH vitamin D deficiency in RA patients as also seen in other countries. The deficiency seen in USA is 43%, in Italy 52% and in Iran 41% [20]. We used two different cut-offs for defining the 25-OH vitamin D deficiency, insufficiency and sufficiency for the accuracy of the results. We observed that RA patients are deficient in 25-OH vitamin D levels as compared to the controls (45.24% vs. 43.48%). This level was statistically significant between the 25-OH vitamin D deficient RA patients and the 25-OH vitamin D deficient controls (p = 0.013). Independent t-test was used to evaluate this difference.
It is seen that RA patients with high disease activity have lower 25-OH vitamin D levels as those with low activity score [24]. Our study had 69.05% of the RA patients with high DAS28-CRP score while 30.95% with moderate DAS28-CRP scores. We also found that the patients with high DAS score had significantly lower 25-OH vitamin D than with moderate DAS score (p < 0.05). The reason for the lower levels may be due to poor exposure to direct sunlight. Azzeh, et al. [22] has shown the trend of the baseline characteristics in their study participants according to the disease activity. In our study, 25-OH vitamin D levels in RA patients < 30 years of age is less as compared to the patients between 30-50 years of age (48.29 ± 40.84) nmol/l vs. (79.21 ± 55.58) nmol/l as represented in Figure 2. Studies show that younger patients have lower serum vitamin D levels than older patients with RA [21] Vitamin D deficiency has been reported from different parts of the world, particularly in southern countries, where more than 90% of the affected are elderly people. It is also reported that the 25-OH vitamin D deficiency is also frequent in the younger population [42].
Vitamin D is known to induce immunologic tolerance and its deficiency may increase the risk of development of autoimmune diseases such as RA [12] Also, vitamin D has immunomodulatory properties. It appears to regulate the immune response by decreasing antigen presentation, inhibiting the T-helper type-1 (Th-1) cell that is directed against self-antigens. Also, Th1 type cells are a source of potent inflammatory cytokines such as IFN-gamma and TNF-alpha, which are major drivers of RA pathogenesis [43]. Varenna, et al. reported that vitamin D supplementation may be recommended for RA patients for the prevention and treatment of osteoporosis as well as for its possible effects on disease activity [44]. Franco, et al. concluded in a meta-analysis that vitamin D supplementation could possibly reduce rheumatoid arthritis recurrence [45].
Recently, preventive treatment with vitamin D of individuals considered at high risk of developing autoimmune diseases has been proposed [24]. Our findings are of great potential to clinicians as patients may be assessed as failing to respond adequately to disease modification in the presence of 25-OH vitamin D deficiency. This might have major implications for subsequent management, as they may then be subjected to treatment escalation in spite of the absence of any ongoing disease activity. This carries implications at both clinical and financial levels [46]. Hence, clinicians need to be aware of the potential confounding effect of 25-OH vitamin D deficiency in the assessment of RA disease activity using the DAS28 tool and should consider the measurement of 25-OH vitamin D levels in their RA patients.
Thus 25-OH vitamin D deficiency is common in patients with RA, and that 25-OH vitamin D deficiency may be linked to disease severity in RA. As vitamin D deficiency has been linked to diffuse musculoskeletal pain, these results have therapeutic implications. Vitamin D supplementation may be needed for the prevention of osteoporosis and for pain relief in patients with RA. Also, special attention should be given to checking 25-OH vitamin D levels in patients with high disease activity as it affects health status.
The authors wish to thank: The Director and the Management of Sir H. N. Medical Research Society for the Funds, the Scientific Advisory Committee of Sir H. N. Reliance Foundation Hospital and Research Centre for sanctioning the Project and the patients for participating in this project.
There is no conflict of interest between any of the authors.
This study was approved by the Scientific Advisory Committee and the Institutional Ethics Committee of Sir H. N. Reliance Foundation Hospital and Research Centre and informed consent was taken from the patient before the collection of their samples. The study was carried out in accordance with the "Ethical Guidelines for Biomedical Research on Human Participants, 2006" by the Indian Council of Medical Research and the Declaration of Helsinki, 2008.