Objectives: The aim of this study is to determine the usefulness of the low-price digital microscope in the identification of vascular changes in the nail bed of patients with systemic sclerosis and its relationship with autoantibodies involved.
Methods: Nailfold capillaroscopy (NFC) was performed with a digital microscope at 40x, 200x magnification in 12 patients with systemic sclerosis (SSc), of which seven are limited cutaneous systemic sclerosis (lcSS), five are diffuse cutaneous systemic sclerosis (dcSS) and twelve controls. The images were captured and stored in the computer for later analysis with the device's software. The parameters of each image were classified into "early", "active" or "late" scleroderma pattern, normal and non- specific non-scleroderma pattern.
Results: The scleroderma pattern was seen in 10 of the 12 patients with SSc of which 8 had an active stage with average density of 5.0 ± 0.9, average dimension 84.9 ± 25.6, presence of abnormalities (any capillary whose tip is not convex) and haemorrhages in all cases. Non scleroderma pattern was seen in 2 patients with systemic sclerosis and in all control patients with average density of 9.6 ± 2.5, average dimension 16.9 ± 4.5 presence of abnormalities in 7.1% and haemorrhages in 21.4%. The late stage was seen in 2 patients with an average density 2.5 ± 0.7, average dimension 32.5 ± 10.6, abnormal morphology present and no haemorrhages. The non-scleroderma pattern is classified into normal and non-specific pattern. The non-scleroderma pattern classified as normal was seen only in controls and the non-specific pattern in 3 controls and 2 patients with SSc with non-significant variable densities and dimensions. ANA was positive in 90% of the patients with scleroderma pattern of which 87.5% of the patients with active stage presented ANA positive and 100% of the patients with late stage had ANA positive. Anti-centromere antibody (ACA) was positive in 50% of the patients with scleroderma pattern divided equally into active stage (50%) and late stage (50%). Anti Scl-70 was positive in 10% of the patients with scleroderma pattern, 12.5% with active stage and 0% with late stage. In the group of non-scleroderma pattern only 7.1 % of the patients had ANA positive, 7.1% anti Scl-70 positive and 0% ACA positive.
Conclusions: NFC by digital microscope is useful to determine the different patterns that can be observed in systemic sclerosis with a good quality. The active and late-stage scleroderma pattern was predominant in our study population and seems to be related to the positivity of ANA and anti-centromere antibodies.
Digital microscope, Nailfold capillaroscopy, Systemic sclerosis
Nailfold capillaroscopy (NFC) is a non-invasive and useful tool that permits Rheumatologists to evaluate microvascular abnormalities found in Raynaud's Phenomenon (RP) frequently associated to systemic connective tissue diseases (SCTD) in particular patients with systemic sclerosis (SSc) [1-3]. Systemic sclerosis is a rare chronic immune-mediated disease causing skin and internal organ fibrosis, and microvascular damage [4-6] characterized by ischaemia, loss of peripheral capillaries, revascularization and lack of capillary repair [7]. NFC allows us to distinguish between primary (idiopathic) to secondary (disease associated) RP and classify the microvascular abnormalities as "early", "active" and "late" in scleroderma pattern or non-scleroderma pattern which is divided into normal and non-specific [8,9]. The gold standard device for NFC is the digital videocapillaroscope [10,11], that despite its advantages it is an underused piece of equipment or is not available in developing countries due to high cost of equipment and shipment.
There are specific antibodies that are related to manifestations and complications of the disease such as anti-centromere antibody (ACA) that predict subcutaneous calcinosis, pulmonary arterial hypertension (PAH), and gastrointestinal involvement, antibodies against DNA topoisomerase I (ATA) also known as anti Scl-70 that can predict the development of interstitial lung disease (ILD) and digital ulcers but appear to be protective against PAH [12-14].
The objective of this study is to determine if the low-price digital microscope allows us to accurately identify the different patterns and alterations in the nail bed microvasculature in patients with systemic sclerosis and its relationship with autoantibodies involved.
Between May 2020 and April 2021, 12 patients were consulted in AGAR (Asociación Guatemalteca Anti-enfermedades Reumáticas) clinic from the rheumatology outpatient department. All the patients fulfilled the 2013 American College of Rheumatology classification criteria for SSc [15]. We classified the patients with limited cutaneous SSc (lcSS) and diffuse cutaneous SSc (dcSS) according to the LeRoy, et al. criteria [16]. All patients were older than 18-years-old and met the inclusion and exclusion criteria (Table 1). The control patients were selected according to the sex and age of the patients with SSc with a difference of (± 2 years). Data confidentiality was guaranteed and the ethical principles established by the HELSINKI Declaration were respected.
Table 1: Inclusion and exclusion criterias. View Table 1
A digital microscope is an inexpensive instrument that can be connected through the USB port in a computer. The software included in the device must be installed in order to capture, film, store images and perform simple capillary measurements. To be able to carry out the measurements it needs to be calibrated with the microscope micrometer calibration ruler that it's included in the device. The instrument is capable of magnifying up to 40x - 200x. It is not difficult to operate and only requires a short period of training. In this study we used the Jiusion 8 LED USB 2.0 digital microscope, Mini camera with OTG adapter and metal stand connected to a windows 10 Home 64 HP laptop.
The patients filled out a complete clinical evaluation and afterwards the NFC was performed with a digital microscope at 40x, 100x and 200x magnification. The images were captured and stored in the computer for later analysis. Images were obtained from all of the fingers (except thumbs) after a previous hand washing and the application of a drop of immersion oil on each capillary bed. Two images were taken of adjacent 1mm fields in the middle of the nail bed with 200x magnification. These images were analyzed with the device software to make the correct measurement of capillary density and dimension. The parameters of each image were classified into "early", "active" or "late" scleroderma pattern and normal and non-specific non-scleroderma pattern according to the "Fast Track algorithm" EULAR Study Group on Microcirculation in Rheumatic Diseases (EULAR SG MC/RD) modified by Smith in 2020 into an algorithm easy to use in clinical practice [9,17]. All the doctors who analyzed and took the images are trained in capillaroscopy and the use of the device.
The P-values of the statistical tests were calculated with the Student's t-test or chi-square and were obtained with the StatXact software of Cytel Studio version 10.0, for small sample sizes. All parametric data were analyzed by Student's t-test and non-parametric data were analyzed by Chi-square test. Comparisons with P-value of < 0.05 was considered statistically significant.
In this study, a total of 12 patients with systemic sclerosis (all female) and 12 age- and sex-matched healthy controls were evaluated with NFC. Demographic, clinical, capillaroscopic and laboratory characteristics of the patients and controls are shown in Table 2. The mean age of patients and controls was 51.0 ± 15.3 and 52.8 ± 16.5 years, respectively. The mean time of illness was 12.7 ± 8.5, 9.4 ± 7.3 for lcSS and 17.4 ± 4 years for dcSS taking into account the onset of symptoms up to the moment of performing the NFC. Raynaud's phenomenon was present in all patients and absent in controls (P < 0.0001). Within the characteristics of the NFC, the capillary density in patients with SSc was lower compared to controls, 4.9 ± 1.6 and 10.1 ± 2.4 capillaries per millimeter respectively. The mean size of capillaries in patients with SSc was much greater than in control patients, 65.3 ± 35.7 and 16.4 ± 4.1 (μm) respectively. Abnormal morphology was found in 91.7% of patients with SSc and no abnormal shapes were found in control patients. Haemorrhages were present in 83.3% of patients with SSc and 8.3% in control patients. Most of the patients (83.3%) had a positive ANA in dilution of at least 1:80. Auto-antibodies anti-Scl-70 and ACA were present in 16.7% and 41.7%. Characteristics of the scleroderma pattern and non-scleroderma pattern are shown in Table 3. 10 of the 12 patients with SSc had a scleroderma pattern of which 8 had an active stage with an average density of 5.0 ± 0.9, average dimension 84.9 ± 25.6 and presence of abnormalities and haemorrhages (Figure 1a). The late stage was seen only in 2 patients with an average density 2.5 ± 0.7, average dimension 32.5 ± 10.6, abnormal morphology present and no haemorrhages (Figure 1b). ANA was positive in 90% of the patients with scleroderma pattern (P < 0.0001) of which 87.5% of the patients with active stage presented ANA positive and 100% of the patients with late stage had ANA positive. Anti-centromere antibody (ACA) was positive in 50% of the patients with scleroderma pattern (P < 0.0059) divided equally into active stage (50%) and late stage (50%). Anti Scl-70 was positive in 10% of the patients with scleroderma pattern, 12.5% with active stage and 0% with late stage. The non-scleroderma pattern is classified as normal (Figure 2a) and non specific (Figure 2b). The normal pattern was seen in all control patients and the non specific pattern was seen in3 control patients and 2 patients with SSc with non-significant variable densities and dimensions. In this group of non-scleroderma pattern only 7.1 % of the patients had ANA positive, 7.1% anti Scl-70 positive and 0% ACA positive.
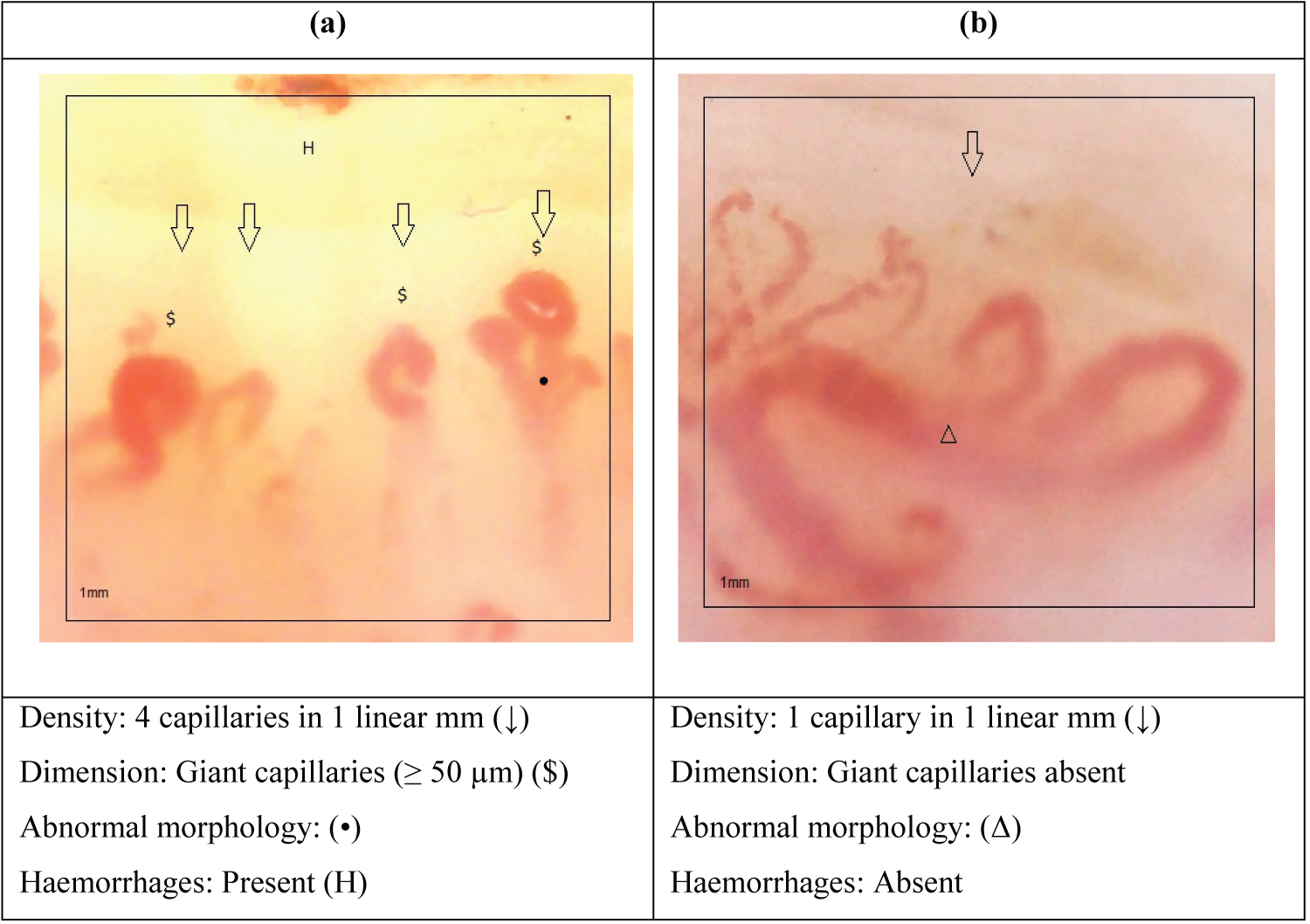 Figure 1: a) "Active" scleroderma pattern; b) "Late" scleroderma pattern.
View Figure 1
Figure 1: a) "Active" scleroderma pattern; b) "Late" scleroderma pattern.
View Figure 1
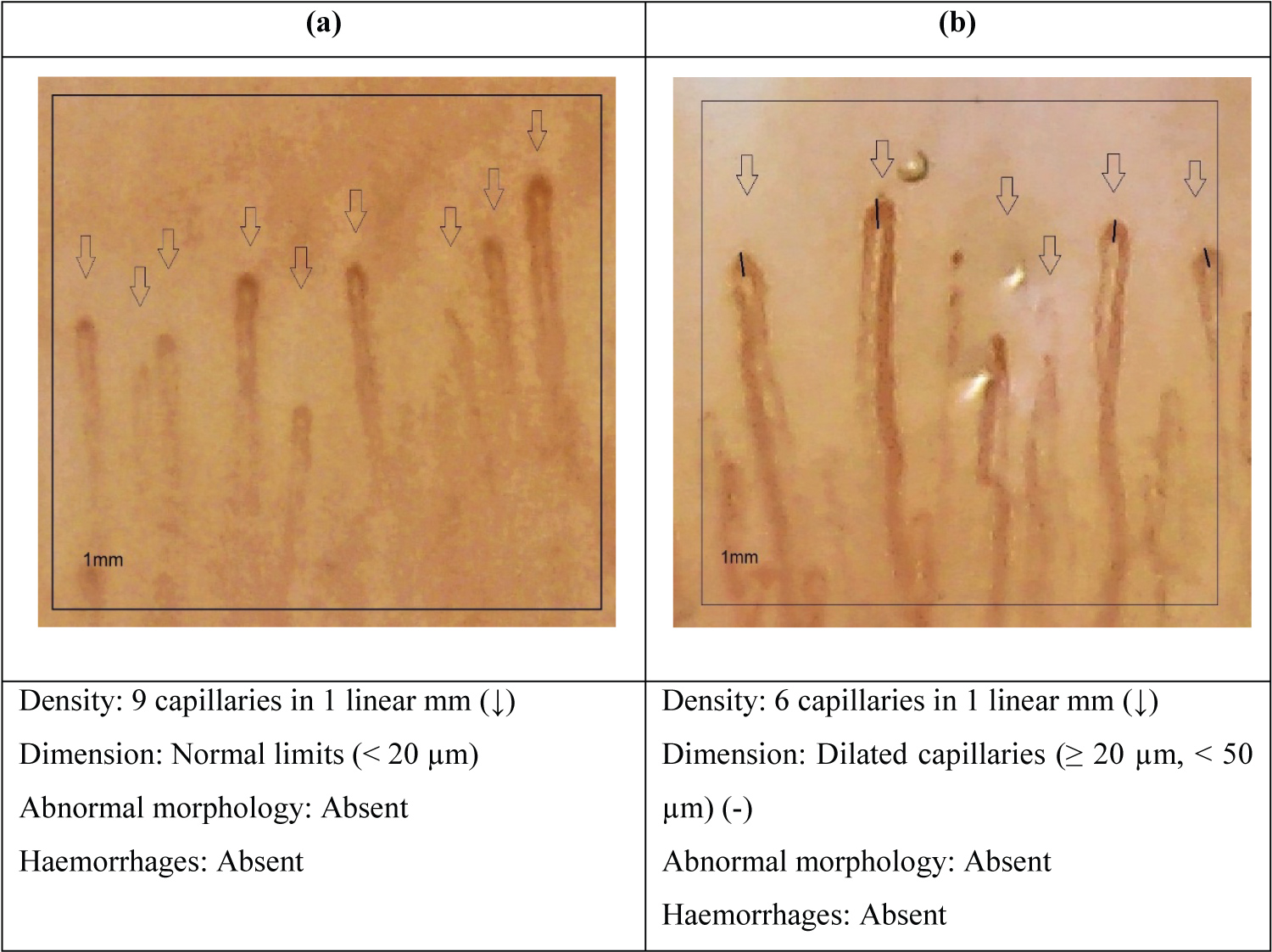 Figure 2: a) Normal pattern; b) Non-specific pattern.
View Figure 2
Figure 2: a) Normal pattern; b) Non-specific pattern.
View Figure 2
Table 2: Demographic, clinical, and laboratory characteristics of the patients and controls. In bold and gray background, the comparisons that present statistical significance (p < 0.05) are highlighted. View Table 2
Table 3: Demographic and clinical characteristics of the patients according to scleroderma pattern. In bold and gray background, the comparisons that present statistical significance (p < 0.05) are highlighted. View Table 3
NFC is currently very useful to differentiate the primary from secondary Raynaud's phenomenon, the latter commonly related to autoimmune rheumatic diseases [1,18]. It is well known that most patients have RP as the first sign in autoimmune rheumatic diseases especially in systemic sclerosis and this can appear several years before developing the disease [19,20]. In SSc, capillaroscopy alterations are included within the ACR/EULAR 2013 classification criteria, granting it 2 points of the 9, necessary for its classification [15]. The videocapillaroscope is the device of choice to perform this examination [10,11], but it continues to be too expensive in our environment to be able to use it with our patients, also considering that most of them have limited economic resources. The digital microscope is inexpensive and easy to use in clinical practice [21,22]. In addition, it is portable and has a simple software to perform measurements which must be installed on a laptop or computer. It is capable of storing images and videos to be analyzed later. With this device it can be magnified from 20x to 200x and allows us to identify all the changes described in several studies with a relatively good quality.
The scleroderma pattern is the most frequent in patients with SSc although it can also be seen in dermatomyositis (DM), mixed connective tissue disease (MCTD), overlap syndromes, undifferentiated connective tissue disease (UCTD) and systemic lupus erythematosus (SLE) [23]. The characteristic most found in SSc is the presence of giant capillaries (≥ 50 μm). There could also be a decrease in capillary density (< 7 capillaries per linear mm), abnormalities in the shape and presence of hemorrhages [9,17].
In our study, all patients with SSc reported RP as part of the initial signs of the disease, and the majority had giant capillaries, decreased density, abnormalities in shape, and hemorrhages corresponding to an active stage of the scleroderma pattern. Only two of the patients with SSc had a late scleroderma pattern stage characterized by capillary density ≤ 3/mm, abnormal morphology and absence of giant capillaries and hemorrhages despite the many years of onset of the disease.
Regarding SSc-specific antibodies, they are known to be indicators of clinical subsets of the disease and can guide the diagnosis and follow-up of individual patients [24,25]. The autoantibodies found in patients with a scleroderma pattern were ANA (90%), Scl-70 (10%) and ACA (50%) and they were found in a higher proportion (except anti Scl-70) compared to patients with a non-scleroderma pattern. None of the controls were positive for autoantibodies.
In this study we determined the usefulness that the digital microscope can have for the performance of NFC in patients with SSc. Capillaroscopy characteristics that include density, size, abnormalities and haemorrhages can be easily identified and can help us in the diagnosis of SSc. We were able to visualize and distinguish between scleroderma and non-scleroderma patterns in our small population with a low-cost device that is easy to use in clinical practice. The positivity of ANA and ACA seems to be related to the active and late-stage scleroderma pattern in patients with SSc. Future studies in different clinical settings with larger numbers of SSc patients and healthy individuals are necessary to clarify the effectiveness of this device and the relationship between autoantibodies and scleroderma pattern.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.