Background: Rabies, a negative strand RNA virus belonging to the genus Lyssavirus has existed since hundreds of years. Available historical texts called it 'an ancient curse'. From Aristotle in fourth century BC to present day, rabies virus has existed in various species of animals. Modern day molecular epidemiology has proven the evolving nature of the virus. The virus showcases considerable genetic plasticity and hence it still exists as a tangible threat in the 21st century.
Text: The 2019 Global Burden of Disease Study revealed a considerable number of Rabies related human deaths. The global strategic plan, proposed by World Health Organization (WHO) was to be canine- rabies free by 2030 (Zero by 30). Adopting this plan, affected countries would come closer to fulfilling Sustainable Development Goal (SDG) 3.3. This goal encompasses the WHO strategy to end epidemics of neglected tropical diseases like Rabies through education, awareness and specific medical treatment. Rabies is mainly concentrated in Asia and Africa but America, Canada and Europe are facing renewed threats via bat rabies. Disease among travelers to Rabies-endemic areas and risk of re-introduction of canine Rabies is a matter of concern. Renewed efforts to tackle Rabies include newer laboratory techniques, novel vaccines and immunoglobulin. Some diagnostic techniques have been standardized internationally. Mouse inoculation tests are being replaced by cell cultures. Specific nucleic acid probes and DNA sequencing techniques are being used for rapid confirmation of the clinical cases. Post-Pasteur vaccinologists have developed next-generation vaccines, overcoming the conventional drawbacks of the old ones. These efforts were gravely hampered by the COVID-19 pandemic in 2020. Unlike other infectious diseases, telemedicine cannot be a solution for animal-bite victims. Anti-rabies clinics were shut down and there were logistical issues where patients could not receive timely post-exposure prophylaxis. There were significant disruption of health care services and major Rabies control targets were wiped out especially in low and middle income countries. There are questions on the affordability, availability and accessibility of therapeutic options in the present day.
Conclusion: Existing in the past and in our present, numerous scientific articles have been written on Rabies. This comprehensive review is one step towards analyzing a unified global effort 'One Health Approach' for achieving zero human deaths by 2030. Elimination of this zoonotic disease in near future will be a test case for World Health Organization. In spite of a pandemic setback, it will help reduce global health inequality and strengthen our fight against neglected tropical infections. It will help in our preparedness for future infectious disease emergencies.
WHO: World Health Organization; PCR: Polymerase Chain Reaction; RIG: Rabies Immunoglobulin; DFAT: Direct Florescent Antibody Test; PrEP: Pre Exposure prophylaxis; PEP: Post Exposure prophylaxis; AIDP: Acute Inflammatory Demyelinating Polyneuropathy
Rabies, a viral zoonotic disease forms part of the ‘Neglected Tropical Diseases (NTD)’ enlisted by World Health Organization (WHO) [1]. According to the study on Global burden of Rabies, it causes approximately 14000 human deaths annually, children below 14 years of age constitute 40% of the bite victims and Africa, Asia bear the burden of maximum cases of canine rabies [2].
Being largely ignored by global funding agencies, rabies prevention programs have hit many roadblocks. Limited resources have been made available to countries across the globe especially the developing ones. WHO, the World Organization for Animal Health (OIE), Global Alliance for Rabies Control (GARC) and their partners have formulated specific targets and a common goal of ‘Zero Human Deaths by 2030’ [3]. These targets have been aligned with the Sustainable Development Goals (SDG) Program, specifically SDG 3.3 [4]. The Covid-19 pandemic hit this renewed combat against Rabies mainly affecting communities with high burden. Hence, accurate scientific information and discussions regarding this largely preventable disease in post-Covid era becomes essential.
Rabies has been the subject of hypotheses and research since its discovery by Louis Pasteur in the 20 th century. Studies on origins of Rabies disease associate human rabies with canine rabies. Since ancient times, dogs have been considered as the main vector of this disease. However with the advent of modern diagnostic and genetic tools, it has been established well without doubt that rabies virus exists in many animal species [5].
The word rabies comes from the Latin word ‘rabere’ meaning ‘to rage’, also known as canine madness or hydrophobia [6]. Aristotle, the famous Greek polymath, in his book ‘History of Animals’ quoted ‘if the rabid dog bites, all the animals bitten become rabid’ [7]. Reference to Rabies occurs in texts from ancient Mesopotamia (circa 2200 BC). The disease was associated with the appearance of Dog Star Sirius in summers when the dogs were prone to spells of madness. In the 1 st century AD, famous Indian text ‘Susrutasamhita’ mentioned this disease with its signs and symptoms [8]. Arab and Persian authors have mentioned rabies in their texts in the 11 th and 15 th centuries. In 1804, Georg Gottfiel Zinke performed an experiment transmitting rabies from rabid dog to normal dog and then to rabbit and hen [9].
Pasteur established the theory of rabies virus being present in the infected animals’ brains. By serial inoculation of the rabies virus in rabbits, he was successful in obtaining a ‘fixed’ virus. He then demonstrated how multiple injections of this ‘fixed’ virus to dogs could render them immune. It was a scientific triumph when the same experiment could be replicated in humans. In July 1885, Joseph Meister, a nine-year-old boy was bitten by a rabid dog. He was inoculated with 13 injections of this crude vaccine by Pasteur. The boy survived and a breakthrough was obtained in the treatment of Rabies infection.
Humans are a ‘dead end’ host in Rabies. Direct person to person transmission has not been documented in literature. However, unusual rare modes of transmission which has been recorded are through infected corneal grafts and solid organs [10]. The donors had died of unsuspecting Rabies and their tissues harbored the virus.
Current scientific knowledge confirms the prevalence of Rabies virus in terrestrial warm blooded mammals and bats. Ancient scriptures mention of a ‘disease of madness’ among wild carnivores but prevalence of Rabies in bats was a relatively recent discovery. Unfortunately, comprehensive data on global epidemiological trends, especially from endemic countries, remains scarce.
Two types of Rabies exist in nature-Urban Rabies, which is transmitted by domesticated animals like dogs (canine), cats and the other, Sylvatic type which involves foxes, raccoons, wolves, jackals and others. In developed nations like the United States, the canine rabies virus variant has been eliminated, but wildlife variants (such as bat, raccoon rabies virus variants) remain [11]. In Asia and Africa, 90 percent of human rabies is through exposure to rabid dogs. India leads the tally in human deaths due to rabies. Prevalence and the status of rabies control programs in the Indian sub-continent are mentioned in detail, later in the article. To give a better understanding, the world-wide prevalence of endemic human Rabies is illustrated in the given World Map (Figure 1). The data has been sourced from WHO Rabies prevalence fact sheet 2021.
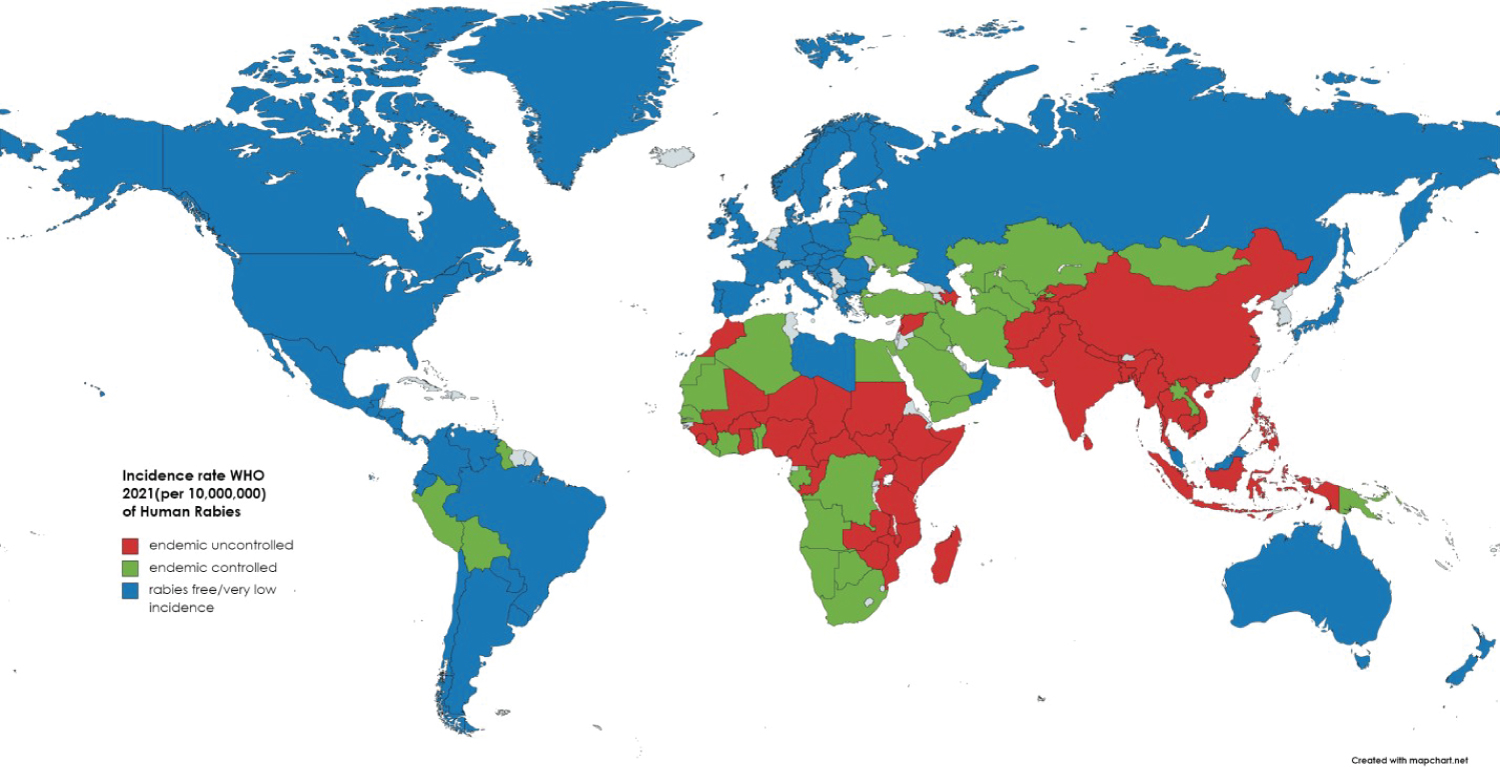 Figure 1: Incidence rate WHO 2021 (per 10,000,000) of Human Rabies.
View Figure 1
Figure 1: Incidence rate WHO 2021 (per 10,000,000) of Human Rabies.
View Figure 1
The rabies virus is a rod or bullet shaped, unsegmented, enveloped RNA virus. Comparative genomic sequencing places it in the family Rhabdoviridae and genus Lyssaviruses. The virus is composed of an internal protein core or nucleocapsid containing the nucleic acid and an outer envelope, a lipid bilayer covered with glycoprotein spikes. The virus genome has 5 proteins. Few of these proteins aggregate in the cytoplasm of virus infected neurons and form Negri Bodies, the pathognomonic histopathological finding in Rabies infection [12].
Human infection almost always occurs via the bite of an infected animal. Non bite infections like contamination of an open wound via scratches/licks and through aerosols have been documented rarely [13].
The virus exists in the saliva of the rabid animal. Initially, the virus may replicate in the skin and muscle tissue around the site of inoculation. This may last for 48-72 hours. The virus then enters the nervous system via transmission across neuromuscular junctions. It moves centripetally along the axons towards the spinal cord and brain. Rapid replication occurs in the brain after which the virus is disseminated centrifugally to many tissues and organs. It primarily resides in the salivary glands, cornea and facial tissue. Replication occurs in the salivary glands due to which constant virus shedding occurs in saliva. The virus may be shed in milk and urine also. The pathophysiology has been depicted in Figure 2.
 Figure 2: Pathogenesis in domestic rabies.
View Figure 2
Figure 2: Pathogenesis in domestic rabies.
View Figure 2
Glycoprotein G is the main component present in the spikes of the viral envelope. These are chiefly responsible for virulence and immunity. These spikes bind to the acetyl choline receptors of the neural tissue stimulating hemagglutination-inhibiting (HI) and neutralizing (protective) antibodies. Therefore, it provides an effective and safe vaccine model. The nucleocapsid protein or the internal viral core protein also induces antibodies. These are called complement fixing antibodies but these are not protective in nature [14].
Eliciting a history of bite or lick/scratches from possible rabid animal is the key in early diagnosis of rabies [15]. The progression of the disease depends on the nature and extent of infection.
The incubation period is mainly 1-3 months though it may vary from few days to years. The incubation period was found to be shorter in those individuals bitten on the head or face. This is probably related to the distance the virus travels to reach the brain. Scientists have divided the course of disease into four categories: Prodrome, acute encephalitis stage, coma and death.
The prodrome is marked by non specific symptoms. In case, history of bite cannot be elicited, it may be extremely difficult to make accurate diagnosis during this stage. Anorexia, fever, headache, malaise and paresthesia/pain at the site of virus entry may be seen. It may last for 2-4 days. Encephalitis occurs when the virus reaches the brain and starts replicating. There may be bouts of extreme hyperactivity along with seemingly normal behavior. Delirium and hallucinations are common symptoms seen. One characteristic feature in patients is termed as hydrophobia or fear of water. In spite of intense thirst, patients dread the sight of water. This is because any attempts to drink result in painful spasms of the pharyngeal muscles. A majority of patients die due to respiratory arrest in the encephalitic stage. It may last for 2-10 days. Some patients progress to paralysis. Rarely, there may be no signs of hyperactivity and there is appearance of paralytic disease from the onset. This kind of clinical presentation can be a diagnostic dilemma for the clinicians. It can be confused with demyelination induced quadriparesis. Acute Inflammatory Demyelinating Polyneuropathy (AIDP) can also have a similar clinical picture. Thus, the clinical spectrum can prove to be one of the most difficult conundrums for the experts. Death is preceded by coma which may last for hours or few days. Unvaccinated individuals with history of rabid bites have 100% fatality.
Until recently, there was minimal focus on laboratory confirmation of Rabies disease. Due to the fatality of the disease, no serious attempt was made on confirming Rabies or treatment except heavy sedation. If a person survived, it was considered a non-rabid infection. In the present day, when survival from established rabies has been documented in rare instances [16], there has been renewed emphasis on making laboratory distinction between Rabies and other encephalitis-causing diseases. Several diagnostic tests are being marketed commercially which prove to be key assets in the battle against Rabies.
The laboratory diagnosis of Rabies dates back to the year 1903-5 when the microscopic detection of Negri Bodies marked a milestone in the fight against Rabies [17]. Aldechi Negri demonstrated these intra-cytoplasmic inclusion bodies in the brains of rabid animals. Unfortunately, there is lack of absolute confirmation as Negri bodies may be absent in about 20% of suspected Rabies. In 1936, Webster and Claw successfully grew the Rabies virus in tissue culture [18]. We present an overview of the various methodologies used in the diagnosis:
1. Virus detection- Intracerebral inoculation of suspected rabid samples in mice is one of the conventional methods [19]. Samples can be saliva, cerebrospinal fluid or urine from patients which can be collected at multiple intervals. Chances of isolation are better early in the disease when neutralizing antibodies have not been produced. The inoculated mice are observed for signs of illness and their brains are examined postmortem for Negri bodies (28 days post-inoculation). This test is not in the list of World Health Organization’s recommended tests for Rabies. There is delay in results due to the 28 day waiting period. Negri bodies might be absent in many cases. Additionally, animal testing facilities are required. There might be potential ethical conflicts.
2. A more rapid and sensitive method is the isolation of virus in tissue culture cell lines [20]. Many commercially available cell lines arising from neural ectoderm are sensitive for the Rabies virus. One of the commonly used is the Neuroblastoma cell line. Samples are inoculated into these cell lines and repeatedly examined under immunofluorescence. Cytological changes patho gnomonic of Rabies start appearing as early as 3-4 days post-inoculation. Unfortunately, this test is confined to research laboratories as they are labour-intensive. Stringent bio-safety protocols have to be adhered along with the requirement for fluorescent microscopy and trained virologists.
3. Viral DNA detection- Molecular techniques like Polymerase Chain Reaction (PCR) and Real Time Reverse Transcriptase PCR have been utilized as confirmatory tests for Rabies [21]. These are highly sensitive tests applicable to any available suspected rabid samples. Though many commercial versions are available, they are still limited to few laboratories. Primers have been designed for Reverse Transcriptase Loop Mediated Isothermal Amplification (RT-LAMP). This is an attempt to improve the molecular diagnosis. Another promising test is the Nucleic Acid Sequence Based Amplification (NASBA) which overcomes the disadvantages of traditional RT-PCR. Sometimes, there is destruction of cell morphology and diffusion of amplified products in the conventional methods. NASBA utilizes three enzymes- reverse transcriptase, T7 RNA Polymerase and RNaseH under isothermal conditions. All the PCR protocols have to be validated initially under stringent conditions. This is essential to prevent cross-contamination and false positive results. The diagnostic handbook by WHO details out explicitly this molecular methodology [22].
Viral Antigen detection- Recommended by WHO and the main diagnostic assay used worldwide, rabies viral antigen test is done using Direct Fluorescent Antibody Methodology (DFAT) [19,23]. The tested samples are corneal smears, skin tissue or saliva ante mortem and brain samples post mortem. The sensitivity and specificity of this test nears 99%. The main limitation is that the test is extremely observer-dependent. At least two observers should spend sufficient time examining the slides under fluorescent microscope.
There are three other tests which appear to be legal alternatives to DFAT [21]. Though they are less sensitive and specific, they can be used in diagnosis of Rabies. The results obtained have been variable hence a confirmation should always been done. These are
Rapid Rabies Enzyme Immunodiagnosis (RREID)
Rapid Immunodiagnostic Test (RIDT)
Direct Rapid Immuno-histochemical Test (dRIT)
Serological tests are seldom useful due to late seroconversion in humans. Commercial kits are available for detection of rabies antibodies in animals post-vaccination. Serological evaluation is also mandated in cases where Pre-exposure Prophylaxis is given. Antibody levels should be checked at regular intervals for people at high risk. There are immense complexities surrounding the interpretation of rabies serology. Moore, et al. have highlighted this issue extensively in their recent article [24].
Critical step towards elimination of rabies would be to reduce the high proportion of cases in endemic countries. Owing to the fatality of the disease, preventive immunization or Pre-Exposure Prophylaxis (PrEP) is highly recommended by WHO. The cost-effectiveness of these PrEP programmes has been compiled extensively by various organizations. WHO has formulated models especially for endemic countries where PrEP has been considered part of childhood immunization programmes [25]. An article by Royal, et al. [26] elucidates timely intervention in school going children in India. Latest guidelines and recommended target groups for rabies PrEP can be accessed on the Centers for Disease Control and Prevention (CDC) website [27].
Consequences of rabid animal-human exposure yield results with varying severity. There is interplay of various factors like location of bite on body, severity of wound, quantity of virus inoculated and well-timed post wound management. The wound management includes post-bite wound care, Post Exposure Prophylaxis (PEP) and administration of rabies immunoglobulin (RIG). RIG have a unique way of providing passive protection. They neutralize the Rabies virus before vaccine induced antibodies appear. These are usually prepared from humans who have been immunized against Rabies and have very high titers of antibodies. Equine RIG is also available and equally effective. RIG should always be used alongside Rabies vaccine in previously unvaccinated individuals. There are no contra-indications. Unfortunately, there is a global shortage of RIG especially in developing countries.
The category of exposure (three categories) determines the PEP. This has been elaborated in Table 1.
Table 1: Categories for post exposure prophylaxis. View Table 1
With the contemporary understanding of rabies pathology, it is clear that rabies virus can expertly evade host immunity. Without vaccination, the chances of survival are slim. For elimination of deaths by 2030, vaccination becomes immensely important.
Table 2 gives a detailed description of the timeline of rabies vaccines [28-35].
Table 2: Timeline of rabies vaccine. View Table 2
Table 3 gives an overview of the promising future of rabies vaccines [36-40].
Table 3: Novel vaccine platforms. View Table 3
Covid-19 pandemic has had huge ramifications on public health programs and vaccination campaigns. This is true for many diseases especially Rabies which is included in the ‘Neglected Tropical Diseases (NTD)’ category [1]. With the pandemic cloud hovering, there was a sudden global shift in priorities. Health gains achieved over past few years declined or were wiped out in the shortest period of time.
Surveys, involving 47 countries, were carried out by World Health Organization (WHO) in early 2021 in order to assess current status of Rabies and damage done by the pandemic [41]. These surveys questioned government officials, animal ministry officials, non-governmental organizations, dog shelter officials, animal activists and academia. The major operations which were hit by the pandemic were tabulated. Some of the key ones were:
1. Delay in diagnosis, treatment and patient care.
2. Delay/cessation of surveillance services.
3. Delay in vaccine manufacture, dispatch and allocation especially to resource-poor countries.
4. Discontinuation of monitoring and evaluation services.
5. Re-allocation of human resources and budget towards pandemic control.
6. Absenteeism due to illness, mortality and care-giving responsibilities.
7. Lockdowns, curfews and public transport woes.
WHO has deployed damage control measures and set new target goals to tackle rabies. Various remedial strategies have been formulated which can be accessed in detail on its official website [42]. Robust infrastructure for human rabies prophylaxis has been recommended to prepare for any future pandemics. Gongal, et al. have highlighted many cost-effective measures in their study on Rabies post exposure prophylaxis impacted by Covid-19 [43].
Rabies-endemic countries need a proper road map and resources to tackle this preventable disease. It is definitely going to be a challenge for them to stretch their already-bulging health care facilities post 2020.
As it is common knowledge, India is endemic for Rabies and accounts for about 35% of the total deaths due to canine rabies [44]. This zoonotic disease is widespread all across the sub-continent except in the islands of Andaman-Nicobar and Lakshadweep. India reported a total of 72, 77,523 cases of animal bites in 2019. Unfortunately, there has been an upsurge in the cases after a dip in the year 2021. A record 14.5 lakh animal bites were reported in just initial 7 months of 2022 [45]. According to a recent newspaper article, there has been an alarming increase in rabies case in the Indian state of Kerala [46]. Probably, increased dog aggression and vaccine shortage post Covid- 19 pandemic are some of the reasons. 75% of deaths in India occur in rural communities owing to lack of suitable emergency health care and vaccines [47].
Due to the debate on India’s rabies control measures and the quality-efficacy of vaccines, the National Rabies Control Programme (NRCP) was launched as part of the 12 th 5-year plan (2012-2017). The National Action Plan for Dog-Mediated Rabies Elimination (NAPRE), in accordance with WHO, has declared its target of zero deaths human deaths by year 2030 [48]. There is frugal data available regarding the success rate of this plan. Open data Government website also yields no results [49].
Various scientific articles elucidate fallibility of the rabies control program in India [50-52]. However as the adage goes, ‘Every cloud has a silver lining’. Gibson, et al. has highlighted effective implementation of the rabies control program in the Indian state of Goa [53]. The authors highlight key issues to be addressed:
1. Curbing rapidly growing population of stray dogs.
2. Increasing surveillance, both active and passive.
3. Targeting poor vaccination rates in canines.
4. Maintaining consistency in policy formulation and implementation.
Rabies control is still not seen as a top public health investment project in India. There is an urgent need for a political, social and scientific road-map to conquer this vaccine-preventable disease.
Diagnosis of rabies is complicated and largely dependent on history of contact. Laboratory tests are not routinely available and only confirm clinical suspicion. Vaccines and immunoglobulin are expensive and scarce, especially in Asia and Africa. To re-iterate the idiom ‘catching the bull by its horns’; approach to elimination of rabies requires drastic measures. ‘One Health Approach for Zero human deaths’ cannot be achieved without collaboration that cuts across boundaries of human research, animal behavior and administrative will. Only then, the future generations will read about Rabies as a disease of antiquity eradicated in the 21 st century.
The authors declare no potential conflict of interests.