Tigecycline gained large popularity due to its efficacy against multi-drug-resistant organisms. Tigecycline has a good safety profile, and its most encountered side effects are nausea, vomiting and diarrhea. However, tigecycline has been associated with a mild and transient hepatotoxicity. Extreme hyperbilirubinemia and jaundice are rare side effects of tigecycline that have not been reported in clinical trials.
69-year-old male had been treated with high-dose tigecycline for severe hospital-acquired pneumonia caused by multi-drug-resistant Acinetobacter baumannii. After completion of the treatment, the patient became jaundiced with a significant increase in liver enzymes, especially ALP and total bilirubin (10- and 5-fold increases, respectively). The patient underwent extensive investigations to identify the cause of jaundice, including viral serology, auto-immune work-up, abdomen ultrasound, and MRCP, which were all unremarkable. The diagnosis of tigecycline-associated DILI was reached after the exclusion of all other causes. The patient was treated with ursodeoxycholic acid, and his symptoms improved with a declining trend in ALP and total bilirubin on the 25th day after tigecycline treatment. The normalization of liver enzymes and resolution of symptoms occurred after almost 37 days of treatment with tigecycline.
This case highlights the importance of recognizing tigecycline, as a possible cause of severe cholestatic liver injury and jaundice.
Tigecycline, Jaundice, Cholestatic liver injury, Adverse event
ALP: Alkaline Phosphatase; ALT: Alanine Aminotransferase; AMA: Anti-Mitochondrial Antibodies; AST: Aspartate Transaminase; CBD: Common Bile Duct; CMV: Cytomegalovirus; DILI: Drug Induced Liver Injury; EBV: Epstein-Bar Virus; HAP: Hospital Acquired Pneumonia; Hbsag: Hepatitis B Surface Antigen; HCV: Hepatitis C Virus; ICU: Intensive Care Unit; IgM: Immunoglobulin M; INR: International Normalized Ratio; LKM-1: Liver-Kidney Microsome Type 1; MDR: Multi-Drug-Resistant; MDRO: Multi-Drug-Resistant Organisms; MRCP: Magnetic Resonance Cholangiopancreatography; MRSA: Methicillin-Resistant Staphylococcus Aureus; RUCAM: Roussel Uclaf Causality Assessment Method; TB: Total Bilirubin; UNL: Upper Normal Limit; VAP: Ventilator Associated Pneumonia; VRE: Vancomycin-Resistant Enterococci;
Seventeen years ago in 2005, tigecycline was introduced as the first antimicrobial agent of the glycycline family. It was approved for the treatment of complicated skin and soft tissue infection, complicated intra-abdominal infection and community acquired pneumonia [1]. Tigecycline is a broad-spectrum antibiotic and has exhibited excellent activity against resistant organisms, such as methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant enterococci (VRE), and many multi-drug-resistant organisms (MDRO) [2]. This has driven the wide use of tigecycline in clinical practice. Due to its good reputation, tigecycline has been used off-label to treat infections such as ventilator associated pneumonia (VAP), hospital acquired pneumonia (HAP) and bacteremia due to MDRO [3]. Intravenous injection is the only available form. The standard, recommended dose is 100 mg loading dose followed by 50 mg twice daily [1]. An off-label high dose (a 200 mg loading dose, followed by 100 mg twice daily) has also been used for the treatment of severe infection [4].
Tigecycline can be used safely in patients with renal impairment of any degree, and in patients with mild-moderate hepatic impairment (child A and B cirrhosis). Use with caution is recommended in patients with advanced hepatic disease (child C cirrhosis), with a reduced dose regimen (100 mg initial dose followed by 25 mg, twice daily) [1].
Tigecycline has a good safety profile. It is a tetracycline derivative and shares with this class its most common side effects of nausea, vomiting and diarrhea [2]. Similar to tetracyclines, hepatotoxicity has been reported with tigecycline use [1]. Tigecycline-associated hepatotoxicity is known to be mild and self-limiting and does not lead to the discontinuation of therapy. However, severe cholestatic hepatitis with clinical jaundice has been very rarely attributed to tigecycline [5]. Here, I present a case of severe hyperbilirubinemia with jaundice induced by high-dose tigecycline.
The patient is a 69-year-old male, known to have multiple comorbidities: diabetes mellitus, hypertension, and ischemic heart disease. Initially, the patient was admitted directly to the intensive care unit (ICU) after he suffered from an acute hemorrhagic stroke, which affected his level of consciousness, and he required intubation. During his stay in the ICU, he developed VAP (ventilator-associated pneumonia) for which he was treated with meropenem and colistin for 7 days.
After stabilization, he was weaned off mechanical ventilation and was transferred to a regular ward with tracheostomy on room air. A few days later, the patient started to show symptoms and signs of chest infection and a chest X-ray revealed evidence of air-space opacity on the right side. Awaiting results of respiratory and blood cultures, he was treated with meropenem empirically. Unfortunately, the patient's condition deteriorated, he became febrile, and his respiratory condition worsened, with an increasing need for oxygen therapy, but blood pressure was stable (BP 135/76). Respiratory culture was positive for multi-drug-resistant Acinetobacter baumannii, which is resistant to carbapenems and has intermediate sensitivity to tigecycline. After consultation with the infectious disease team, the patient was started on high-dose tigecycline (200 mg loading dose, followed by 100 mg, twice daily) plus extended infusion meropenem. Though MDR-Acinetobacter baumannii was sensitive to colistin, the primary physician and infectious disease team refrained from using colistin as the patient was suffering from an acute kidney injury (serum creatinine, 491 mmol/L), which is mostly attributed to the recent use of colistin.
The patient's condition started to improve clinically and biochemically, with a significant improvement in inflammatory markers, and he was weaned off oxygen. Considering the severity of infection and the MDR organism, treatment with high-dose tigecycline plus extended-infusion meropenem was continued for a total of 14 days.
During the treatment period, the patient was monitored through daily routine labs, which included liver profile tests. His baseline ALP (alkaline phosphatase) was mildly elevated (170-200 IU/L), but ALT (Alanine Aminotransferase), AST (Aspartate Aminotransferase) and total bilirubin were within the normal range before and during the treatment. On day 1 after treatment, liver enzymes were noted to be increasing (total bilirubin 52.3 μmol/L, AST 197 U/L, and ALT 101 U/L). The elevation in liver enzymes was progressive and started to become worrisome when the total bilirubin increased to 5 times the upper normal limit (101 μmol/L), and direct bilirubin was 68 μmol/L, along with a 4-fold increase in ALT (190 U/L) and a 10-fold increase in ALP, reaching 2000 IU/L.
On clinical examination, the patient was jaundiced and complaining of pruritis and had dark discoloration of urine, but there was no other sign of acute liver failure.
The patient was not known to have any history of liver disease. The pattern of liver injury was consistent with a cholestatic pattern (R value is 0.2). Abdomen ultrasound showed normal liver echotexture, patent portal and hepatic veins, and a normal gallbladder and CBD diameter with no sign of obstructive jaundice.
At that time, the patient had been treated for infection, and no new drug had been administered (other than recent treatment with tigecycline and meropenem). To complete the work-up for acute liver injury, further blood investigations, including HBsAg, HCV antibodies, EBV (Epstein-bar virus) IgM, CMV (cytomegalovirus) IgM, AMA (anti-mitochondrial antibodies), anti-smooth muscle antibodies, and anti-LKM-1 (liver-kidney microsome type 1 antibodies), were requested and were all negative.
Gastroenterology team advised the application of MRCP (magnetic resonance cholangiopancreatography), which is more sensitive, to rule out obstructive jaundice, and it was also unremarkable. After extensive investigation, tigecycline was brought to our attention as a possible culprit, and tigecycline-induced liver injury was the most likely diagnosis.
The patient's liver profile was followed daily, along with INR and albumin. Ursodeoxycholic acid (dose 250 mg, twice daily) was also used for symptomatic treatment. On day 25 after treatment with tigecycline, the liver profile started to improve (total bilirubin 47 μmol/L, ALP 491 U/L, AST 97 U/L, and ALT 100 U/L). Complete recovery (normalization of total bilirubin, ALT, and AST) occurred on day 37 after the treatment of tigecycline.
Tigecycline has demonstrated an evident activity against many multi-drug-resistant organisms, which made it an appealing antimicrobial choice to treat nosocomial infections even for off-label indications and with off-label high doses [6]. In this case, high-dose tigecycline was the most appropriate choice as the patient had severe hospital-acquired pneumonia caused by a resistant organism (MDR-Acinetobacter baumannii) that is only sensitive to colistin, which could not had been used due to the presence of acute kidney injury [7].
All the clinical trials that have investigated the efficacy and safety of tigecycline have shown a very good safety profile. Gastrointestinal side effects, such as nausea, vomiting, and diarrhea, were the most commonly reported adverse events [8]. Tigecycline was reported to cause elevation in liver enzymes in 2% to 5% of patients. The elevation in liver enzymes was mild and transient with no need for intervention, and also did not lead to the discontinuation of treatment [5,8]. Around 7000 patients had been included in these trials, but jaundice was not reported as an adverse event [5,9]. Cases with serious adverse effects, such as acute pancreatitis, severe liver dysfunction and jaundice, started to appear post-marketing [1].
Recent studies have focused on assessing the safety of tigecycline and its hepatotoxicity. Two large retrospective studies that included more than 1200 patients estimated the incidence of tigecycline-associated drug-induced liver injury (DILI) to range from 5.7% to 10.3% [10,11], which is higher than what had been reported previously in clinical trials (2-5%) [5]. Similar to this case, both studies showed that the majority of patients had a cholestatic pattern of liver injury (75-85%). More than 80% of cases were mild (grade 1-2); only one case in the study of Shi X, et al. [10] developed grade 4 liver injury (total bilirubin ≥ 10-fold the UNL (170 μmol/L)) with tigecycline treatment. Both studies concluded that the prolonged duration of treatment was associated with a higher risk of tigecycline-associated DILI. Interestingly, results were inconsistent regarding a high dose (100 mg, twice daily) being an independent risk factor for tigecycline-associated DILI. Shi X et al. presented a strong association between the dose of tigecycline and the occurrence of liver injury. The incidence of tigecycline-associated DILI in patients with a high maintenance dose was 40% compared to 18% in patients treated with a standard dose for the same duration (Odds Ratio: 1.028; p = 0.002) [10]. On the other hand, Geng, et al. [4] and Chen, et al. [12] showed that high-dose tigecycline was associated with better survival outcomes and slightly higher, but not statistically significant, elevation in liver enzymes compared to a standard dose [4,12]. Similarly, another study reported no difference in the rate of abnormal liver function between a high and standard dose of tigecycline (19.5% vs. 17%, respectively) among critically ill patients with multi-drug-resistant infections [13].
These results were consistent with the results of a meta-analysis that assessed the efficacy and safety of high-dose tigecycline and included 17 studies and approximately 1000 patients. Authors concluded that high-dose tigecycline resulted in a higher clinical cure rate and a lower all-cause mortality rate, with no significant increase in liver injury compared to standard-dose tigecycline [6]. It is worth mentioning that most of these studies were retrospective, with relatively small sample sizes and large room for bias.
The cause of elevated liver enzymes in this patient was most likely DILI (drug-induced liver injury) [6]. The elevation in ALT was ≥ 5 times the upper normal limit (UNL), along with a ≥ 10 times increase in ALP, and a ≥ 5-fold increase in total bilirubin, as shown in Figure 1 and Figure 2. DILI was most likely induced by tigecycline (updated RUCAM score of 6, probable) [14]. The timing of occurrence of liver injury and the clinically apparent jaundice constituted the main challenge in this case. The elevation in liver enzymes occurred after completing the treatment with tigecycline, which is not typical and drove the treating team to think of other possible diagnoses, such as obstructive jaundice and acute hepatitis. Moreover, tigecycline-associated liver injury is known to be mild, transient and asymptomatic, while this patient showed symptoms of jaundice and pruritis.
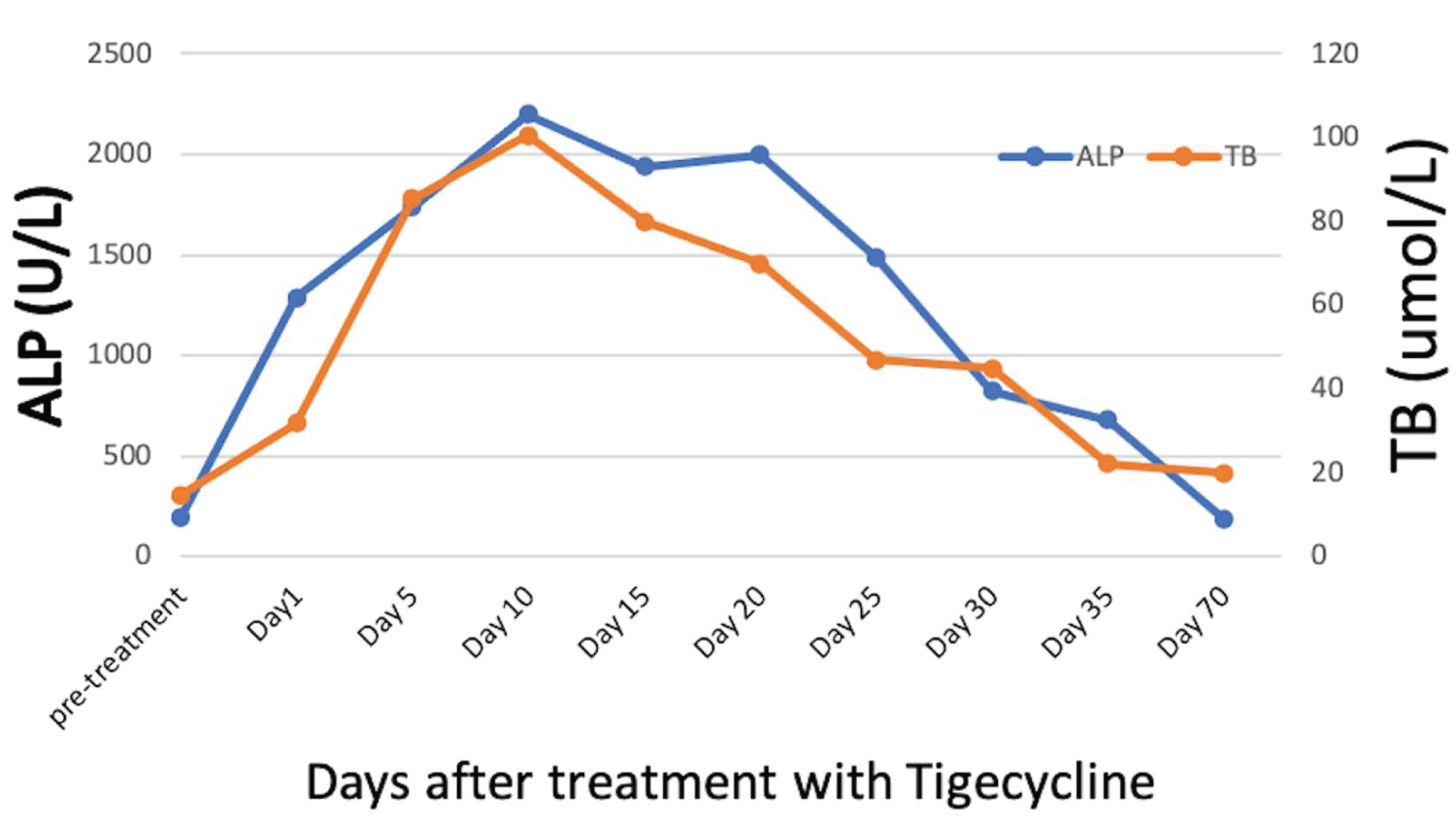 Figure 1: Trend of alkaline phosphatase (ALP) and total bilirubin (TB).
Figure 1: Trend of alkaline phosphatase (ALP) and total bilirubin (TB).
Note: ALP reference range [39-114 U/L], TB reference range [3.4-22.1 umol/L].
View Figure 1
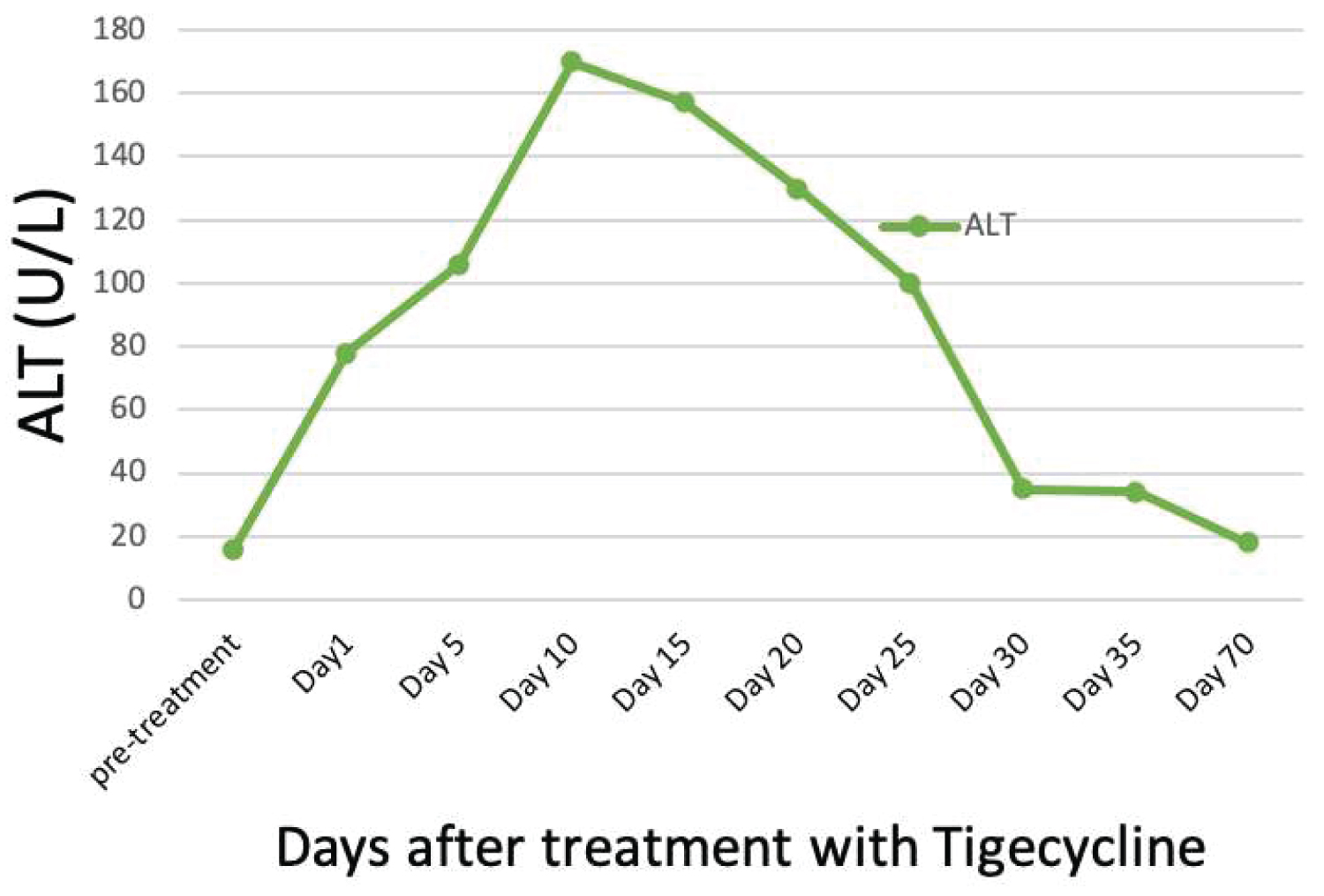 Figure 2: Trend of alanine aminotransferase (ALT).
Figure 2: Trend of alanine aminotransferase (ALT).
Note: ALT reference range [7-44 U/L].
View Figure 2
Reviewing the literature, only one other case of tigecycline inducing severe liver dysfunction with extreme hyperbilirubinemia was reported by Liang, et al. [15]. They reported an extremely high level of total bilirubin, almost 7-fold the UNL (136 μmol/L) in a patient treated with a standard dose of tigecycline. Unlike this case, the elevation in liver enzymes occurred during treatment with tigecycline, which made it easier to attribute it to tigecycline and take the action of reducing the dose and then withdrawing tigecycline. This also spared the patient and physician the time and cost of requesting further investigations to find the cause of liver injury.
A prolonged duration of treatment (more than 10 days) seems to be the most important risk factor for tigecycline-induced liver injury in this case. It is unclear if the dose of tigecycline is another contributing risk factor, as studies present conflicting results, and both this patient and the patient in the study of Liang, et al. [15] had developed severe, clinically apparent liver injury with the two doses of tigecycline.
The mechanism by which tigecycline causes liver injury is unclear [5]. Tigecycline is mostly not metabolized in the body: 59% is excreted unchanged in bile and stool, and 22% in urine [2]. There was no evidence in the literature on the tigecycline-induced changes in the liver at a histopathology level, until recently, when Shi X, et al. [16] reported a case of tigecycline-induced liver injury in a patient with recent liver transplant (16 days before the event). Although the patient did not show clinical manifestations of liver injury, liver biopsy was obtained to distinguish drug-induced liver injury from rejection after liver transplant. Liver biopsy was consistent with cytotoxic injury, as it showed the microcavitation of liver cells, cholestasis and punctate necrosis. This picture is similar to what had been seen with tetracycline-associated hepatotoxicity.
Interestingly, there was a case report of two patients who developed prolonged and severe cholestasis (similar to this case) after receiving intravenous tetracycline. Liver histology showed evidence of bile-duct paucity (i.e., reduced number of interlobular bile ducts) [17].
In our case, though the increase in liver enzymes was extreme, considering the frailty of the patient's general condition; the invasive nature of liver biopsy and its complications, such as bleeding; and the preserved synthetic function of the liver, the treating team opted not to proceed with liver biopsy.
The mainstay treatment for DILI is the withdrawal of the offending drug. In this case, the onset of DILI had occurred after completion of the treatment with tigecycline, and the patient started to become symptomatic in the two weeks after the treatment. There is no evidence in the literature on the most appropriate therapeutic approach to tigecycline-associated DILI. Ursodeoxycholic acid has been used for the treatment of cholestatic liver injury, although definitive evidence of its efficacy is still lacking [18]. Although this patient's symptoms and liver enzymes improved while he was on ursodeoxycholic acid, it is unclear if this was due to spontaneous resolution or the effect of ursodeoxycholic acid. It might have a role in mitigating the symptoms or shortening the illness duration. In the one reported case of biopsy-proven tigecycline-associated DILI, the patient was treated with a tapering dose of methylprednisolone over two weeks, which showed a significant improvement [16]. Again, it is difficult to attribute this improvement to steroids or the simple strategy of the discontinuation of tigecycline.
Clinicians should be aware that tigecycline, especially if used for a prolonged duration, can cause severe liver injury and even jaundice. Moreover, this report emphasizes the importance of following the liver enzymes of patients treated with tigecycline, especially if treated with a high dose or for a prolonged duration.
No conflict of interest.
None.
This manuscript had been prepared and written by single author; Sarah Ali Althomali.