Acinetobacter species are associated with high mortality, A. baumannii being associated with a significant number of Hospital acquired infections worldwide. Multidrug resistance makes it challenging to control or treat these infections. Aim of this study is to evaluate the risk factors and compare the clinical outcomes of Acinetobacter infections in a tertiary care hospital, in COVID and non COVID patients.
A retrospective observational study was conducted. A list of all cases with positive cultures of A. baumannii over a period of one year (January 2020 and December 2020) was retrieved from the microbiology department, using hospital database. The risk factors, antibiotic resistance and clinical outcomes were studied.
94 cases of A. baumannii infections were studied. 89 were caused by Multidrug resistant A. baumannii including carbapenem resistant A. baumannii (CRAB) (92.7%). All were sensitive to Colistin. 28 patients had COVID-19 infection with a mortality rate of 85.7% (p = 0.009). Various risk factors associated with higher mortality were increasing age, Intubation (p = 0.004), male sex (p = 0.023), invasive procedures (p = 0.0001) and carbapenem resistance (p = 0.033).
COVID-19 infection increased the risk of Acinetobacter infections. Carbapenem resistance and invasive procedures were associated with higher mortality rates. Judicious use of antibiotics with proper infection control practices and early diagnosis could be pivotal in preventing prevalence of Acinetobacter infections in the current pandemic situation.
Acinetobacter is a gram-negative, aerobic coccobacillus. Acinetobacter baumannii are important Gram negative opportunistic bacterial pathogens that are responsible for 2-10% of all Gram negative hospital infections. Contrary to other species of the Acinetobacter genus, which are frequently isolated from the soil, water and animals [1], A. baumannii is found almost exclusively in the hospital environment, particularly in intensive care units (ICUs) [2].
Factors associated with Acinetobacter pathogenesis include: cell-surface hydrophobicity, which helps bacterial adhesion; production of slime polysaccharides, which are toxic to neutrophils; production of verotoxins (which block protein synthesis and cause cell death); and the presence of siderophores and outer membrane proteins, which induce apoptosis of epithelial cells [3].
A. baumannii specifically targets moist tissues such as mucous membranes or areas of the skin that are exposed, either through accident or injury. Skin and soft tissues infected with A. baumannii initially present with a "peau d'orange" appearance (similar to the skin of an orange) followed by a sandpaper-like presentation which eventually gives way to clear vesicles on the skin. In areas of skin disruption hemorrhagic bullae can be seen, with a visible necrotising process followed by bacteraemia. If left untreated, this infection can lead to septicaemia and death [4]. Pneumonia may pose a threat to those patients who require mechanical ventilation as A. baumannii has the ability to form biofilms on the surface of the endotracheal tube, which may account for the relatively high levels of colonisation in the lower part of the respiratory tract [4].
A. baumannii has an ability to effectively escape antibiotic treatments. Multi-drug resistance (MDR) in Acinetobacter species is defined as non-susceptibility to at least 1 agent in ≥ 3 antimicrobial categories [5]. The species are becoming increasingly resistant to nearly all routinely prescribed antimicrobial agents including aminoglycosides, fluoroquinolones, and broad-spectrum β-lactams. The majority of strains are resistant to cephalosporin class of antimicrobials [6]. The most common and serious MDR pathogens have been encompassed within the acronym "ESKAPE," standing for Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa and Enterobacter spp [4]. Major risk factors for the acquisition of A. baumannii include antibiotic usage, especially β-lactams-the most commonly used drugs to treat infections caused by important pathogens which cause a variety of diseases in humans and animals. The second most common risk factor is mechanical ventilation, while other risks include surgical wound infections and invasive procedures such as central venous or urinary catheters [7]. As A. baumannii mainly affects immune-compromised and critically ill patients, it has become one of the most important pathogens, particularly in the intensive care unit (ICU) [8].
Coronavirus disease 2019 (COVID-19), caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), quickly spread across the globe and affected more than 150 nations, after its recognition in Wuhan, China, in December 2019. As of 3rd January 2022, there were 290 million confirmed cases of COVID world-wide, with 5.44 million deaths according to the COVID-19 Update for that date [9]. COVID-19 disease symptoms are diverse and may have varied manifestations among patients. It can cause alveolar damage, leading to hypoxia and acute respiratory distress syndrome [10].
SARS-CoV-2 virulence factors interact with the lungs and evoke an immune response. These interactions may compromise innate immunity at several levels resulting in increased bacterial attachment, growth and dissemination. Viral infection may uncover bacterial receptors mediating bacterial attachment [11]. Co-infection may result in an exuberant inflammatory response. It is also plausible that the type of immune response induced by SARS-CoV-2 may enable bacteria to flourish in the lungs. On the other hand, bacterial colonisation may predispose to SARS-CoV-2 infection because the innate immune host defense may be down-regulated enabling virus survival, growth and pathology. Co-infection may exacerbate the tissue damage; and the exuberant inflammatory response may further amplify the lung damage triggered by SARS-CoV-2 [11].
Hospitalization of COVID-19 patients, especially in intensive care units (ICUs), predisposes them to severe consequences such as hospital-acquired infections (HAIs) and/or secondary infections [12]. For example, COVID-19 patients admitted to ICUs with severe pulmonary symptoms can require the use of mechanical ventilation as part of supportive care. This use of mechanical ventilation can lead to ventilator-associated pneumonia (VAP), especially with multidrug-resistant bacteria such as A. baumannii [12].
Lescure, et al. (2020) identified A. baumannii as the responsible agent in a COVID-19 patient of Ventilator Associated Pneumonia (VAP) [13]. Co-infection with A. baumannii secondary to SARS-CoV-2 infections has been reported multiple times in literature during the COVID-19 pandemic including Wuhan (China), France, Spain, Iran, Egypt, New York (USA), Italy, and Brazil [12].
In this study, we tried to analyze the various risk factors, the antibiotic resistance and the recovery profiles of patients with Acinetobacter infections admitted to a tertiary care hospital in South India.
A retrospective observational study was conducted at a tertiary care hospital. The hospital is equipped with 8 ICUs (including 2 surgical ICUs). A list of all cases with a positive culture of A. baumannii over a period of one year (between January 2020 and December 2020) was retrieved from the microbiology department, using computerized hospital database. A total of 94 patients tested positive for Acinetobacter infection. 92 of 94 patients were in-patients.
A. baumannii clinical isolates were recovered from different specimens including endotracheal tube, blood , urine , body fluids including drain fluid, tissue fluid, ascitic fluid, bile , wound swabs, bronchial wash and sputum of patients.
Standard microbiological techniques were followed for processing of the samples. Bacterial identification and susceptibility was done by VITEK 2 compact system. Identification was done by Gram Negative (GN) cards and N281 cards were used for antimicrobial susceptibility testing (AST), as per manufacturer's instructions. Minimum inhibitory concentrations (MIC) of antimicrobials were determined and interpreted. All COVID-19 infections were confirmed positive by Reverese Transcriptas Real-Time Polymerase Chain Reaction test.
Stratification of the patient samples according to the primary specialty of admission was done. Out of a total of 94 samples, 25 samples were from patients who were admitted primarily into ICU, 25 samples from Medical gastroenterology, 15 from surgical gastroenterology, 9 from pulmonology, and the rest were from cardiology, nephrology and urology, orthopaedics and post liver transplant patients. 82 patients were treated in ICUs and HDUs during the course of their hospital stay.
Continuous variables were presented as mean ± standard deviation for normal distribution data and as median (interquartile range) for abnormal distribution data.
The categorical variables were expressed as percentage frequency distribution. Mann-Whitney U test, median test, chi square and Fishers exact test were used in the analysis. A P value < 0.05 was considered as statistically significant.
The risk of mortality based on risk factors was estimated by step wise logistic regression.
All statistical analyses were performed using the statistical package for social sciences (SPSS 21st version) and MedCalc software.
A. baumannii infections are responsible for a significant number of nosocomial infections worldwide. Multidrug resistance makes it challenging to control or treat these infections. Aim of this study is to evaluate the risk factors and compare the clinical outcomes of Acinetobacter infections in a tertiary care hospital, in COVID and non COVID patients (Table 1).
Table 1: Univariate Analysis between COVID and Non-COVID Groups. View Table 1
Medical records of all patients with Acinetobacter infection were retrieved to study the outcomes and risk factors. When the 94 cases were analyzed, the male: female ratio was 76:18. In COVID cases it was 25:3 (M: F), while in non-COVID cases, it was 51:15(M: F). 75% of male patients in the study group succumbed and the mortality rate for female patients was 44%. Male patients had a higher risk of mortality with p-value of 0.023 and Odd's ratio of 3.71 (Table 2). The median age of the patient with Acinetobacter infection was 55 (Inter Quartile Range of 44-67). The median age in patients with COVID and non-COVID infections was 60 years and 54 years respectively. In > 55 years age group, mortality was 75%, while in < 55 years it was 61.9%. 61.5% of discharged patients were of < 55 years age group.
Table 2: Results of Univariate Logistic Analysis. View Table 2
The median time from admission to diagnosis was 7.5 days (p = 0.013). 60.7% mortality was observed when the duration from admission to diagnosis was < 7.5 days while 69.2% of discharged patients had > 7.5 days from their admission to diagnosis. The median duration of hospital stay was 15 days. The relationship between duration of hospital stay and outcome was not statistically significant (p = 0.745) (Table 2).
The study included patients with COVID infection. There were 28 patients who were COVID positive in the study group, 25 of whom received Remedesivir during their course of hospital stay and all of them were treated with steroids. There was a significant association between COVID and Acinetobacter baumannii infection with high mortality and a p-value of 0.009 (Table 2).
The mortality rate was 85.7% in the COVID group with 71.42% (20/28) mortality within the first week of diagnosis. 3 patients were discharged and 1 patient was discharged against advice. All 3 of the discharged group had received sensitive antibiotics and on follow up of the 3 patients, one patient reportedly did well, the other succumbed to a cardiac arrest within 3 months of discharge. In non-COVID group, there was a 58% mortality rate with 41.82% mortality in the first week of diagnosis.
The most common sample which tested positive for the infection was retrieved from endotracheal tube (66/94). Intubation was associated with higher infection rates (p = 0.004). Overall 76 patients required intubation including all 28 COVID patients. Other samples included blood (17/94), body fluids including drain fluid/tissue fluid/ascitic fluid/bile (10/94), wound swab (6/94), urine (5/94), bronchial wash (3/94) and pus (2/94). This includes samples testing positive for both ET tube and blood (15/94) (Figure 1).
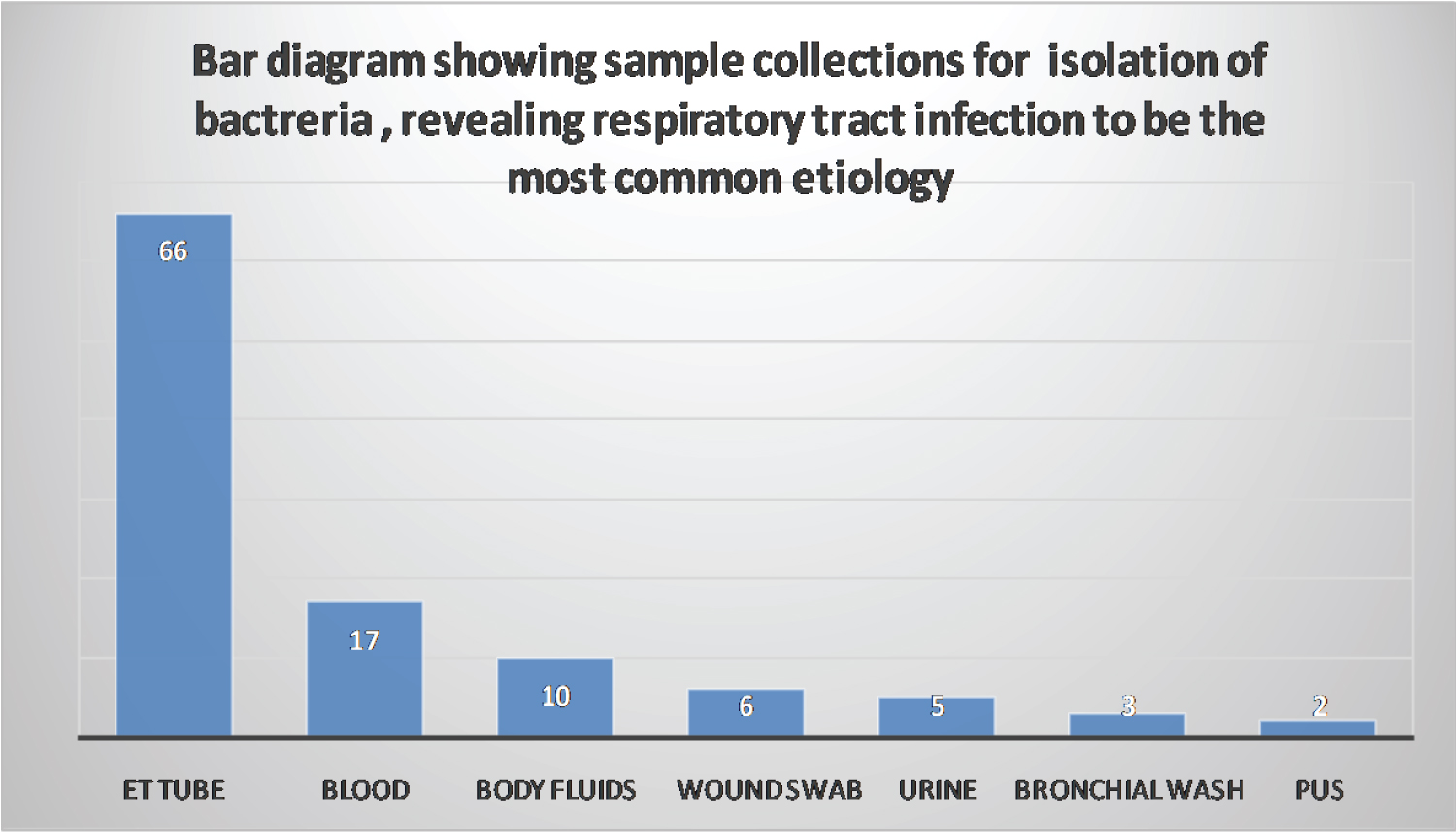 Figure 1: Bar diagram showing sample collections for isolation of bacteria, revealing respiratory track infection to be the most common etiology.
View Figure 1
Figure 1: Bar diagram showing sample collections for isolation of bacteria, revealing respiratory track infection to be the most common etiology.
View Figure 1
Out of 94 cases of A.baumannii infections, 89 were caused by Multidrug resistant A.baumannii (94.68%). All of them were sensitive to Colistin, with variable sensitivity to Tigecycline and Fosfomycin. Carbapenem resistance observed in our study was 92.6%. High mortality was observed in carbapenem resistance group 98.2% (p = 0.033).
In our study, 29 patients had a co-infection and/or secondary infections along with an Acinetobacter infection (Table 3). Most of them, had associated Klebsiella pneumoniae (24/29). Out of the 28 COVID positive cases, 6 (21.4%) cases had Klebsiella pneumoniae with an Acinetobacter infection. All of them succumbed to their illness. Of the 18 non-COVID patients who had a Klebsiella infection , 6 were discharged and 3 patients were discharged against advice, while 9 died. Out of all the deaths associated with A.baumannii infections, 34% were associated with co-infections and/or secondary infections with other organisms but the p value was not significant. The risk of mortality increased in patients with associated co-infections and/or secondary infections, as was observed in our study. Results of multivariate logistic regression revealed that the risk of mortality was more than 3 times when A. Baumannii was associated with co-infections and/or secondary infections versus A. Baumannii alone.
Table 3: Data on Acinetobacter and Associated Infections in COVID and Non-COVID Patients. View Table 3
Previous surgery or an invasive procedure was seen to be a significant risk factor in our study (p = 0.0001) with 91.8% of patients in non-COVID group either undergoing a major surgery (including liver transplantation in 5 patients) or other invasive procedures including tracheostomy, placement of percutaneous drains, intercostal drains, dialysis etc. In COVID group invasive procedures including tracheostomy were performed in 37% of the patients (Figure 2).
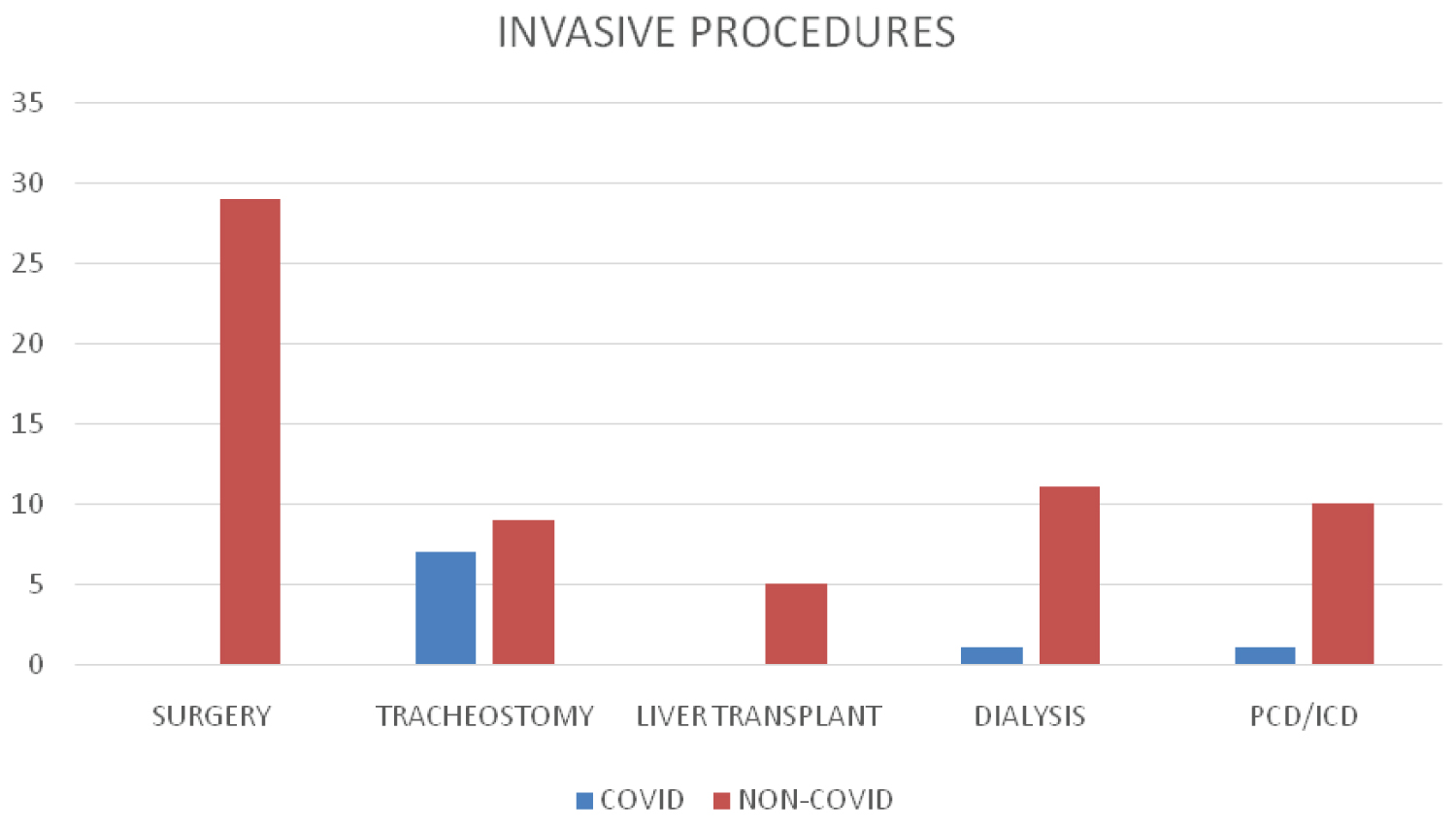 Figure 2: Invasive procedures.
View Figure 2
Figure 2: Invasive procedures.
View Figure 2
The results of multivariate logistic regression revealed that, male sex and COVID infection were the most significant risk factors associated with mortality (Table 4).
Table 4: Results of Multivariate Logistic Regression. View Table 4
Infection due to Acinetobacter species is a major challenge within the health care facilities and the community in general due to their high drug resistance even to the high potent drugs such as carbapenems. Patients in ICU are usually sick, have more invasive procedures, receive prolonged antibiotic therapy and are in close contact with similar patients. A. Baumannii colonizes the respiratory tract, where it can advance to nosocomial pneumonia. Numerous studies have been conducted on prevalence of Acinetobacter infection in Intensive care units [14,15]. In a study conducted by Leão, et al. [16] on critically ill patients in intensive care units, Acinetobacter bacteraemia was associated with higher mortality compared to other pathogens (73% vs. 50%). In another study conducted by Blot, et al. [17], mortality was 42.2%. According to Park, et al. study [18], 87.7% patients were treated in ICUs. In our study, 82 (87.23%) samples were retrieved from patients admitted to ICUs and HDUs with 74.32% mortality.
On analysis of various risk factors, higher age was associated with greater mortality in COVID patients. All patients above 60 years of age with COVID and Acinetobacter infection, had succumbed. In a study conducted by Abdollahi, et al. [19], the mean age of patients was 63.94 ± 13.8 years in COVID patients infected with Carbapenem-Resistant A. Baumannii (CRAB). The mean age was 62.1 years (ranging from 22 to 98 years) in the Hua Zhou, et al. [20] study, 41.2 years in the A. Balkhair [21] study group and in our study the mean age was 52 ± 15.7 years in non-COVID patients affected with Acinetobacter infections.
Compared to a retrospective study conducted by Hua Zhou, et al. [20], over a 60-month study period, 235 of 338 (69.5%) were males while our study had 76 (80.9%) males. Male patients had a higher risk of mortality with p-value of 0.023 and Odd's ratio of 3.71 in our study. 67% of patients with CRAB bacteraemia were males in another recent study conducted by A. Balkhair [21].
The median hospital stay until isolation of A. baumannii was 7.5 days, and the median total hospital stay was 15 days in our study. In patients whose time from admission to diagnosis was more than 7.5 days, 69.2% were discharged. The median duration of ICU stay before infection diagnosis was 9 (IQR: 5-13.3) days in a study by Uwingabiye, et al. [22]. The mean (± SD) length of stay (LOS) for the patients was 12.21 (± 6.90) days in a study conducted on COVID patients developing co-infections [23] and 15.8 (± 6.52) in our study.
Antibiotics according to sensitivity profile, were administered in 57.69% (15/26) of survivors, while 70.90% (39/55) of those who died, had also received antibiotics according to sensitivity.
The A.baumannii isolates included 274 (81.1%) Multidrug resistant Acinetobacter baumannii (MDRAB) in the Hua Zhou, et al. [20] study. In a retrospective case series study conducted at a tertiary referral teaching hospital by Almomani, et al. [24], MDRAB were 98.3% and while by Yadav, et al. [25], the MDRAB were 91%. In our study group, 94.68% (89 cases) were MDRAB indicative of increasing antibiotic resistance.
Carbapenems have been the drug of choice for the treatment of infections caused by A.baumannii. However, the number of isolates resistant to these antibiotics has increased in recent years. According to a study conducted by Zhang, et al. [26], the resistance rate rose from 10.0% in 2008 to > 80.0% in 2011. In a more recent study looking at carbapenem resistance of Acinetobacter over a decade, rate of resistance increased from 67% to 86% (2007-2016) [21]. Patients in our study had 93.9% resistance to carbapenems.
Previous studies have shown poor prognosis in carbapenem resistant Acinetobacter infections. In a meta-analysis by Yang, et al. [15], 52.7% mortality was seen in CRAB. In our study the mortality was 71.4% in CRAB infections.
Polymyxins, including colistin, are the essential last-resort treatment for infections caused by CRAB infections. However, with the increased use of colistin in recent years, reports of resistance have also been increasing. This is of great concern, given the limited number of antimicrobial agents available to treat such infections. However, all cases in our study showed sensitivity to colistin but the mortality in these patients was 68.3%. In a study conducted by Lim, et al. [27], 35.5% of colistin-sensitive group died within 30 days of bacteremia. Our study showed higher mortality rates compared to previous studies.
The study included COVID patients and it was seen as a significant risk factor for Acinetobacter infections, with high mortality (p = 0.009). The increasing evidence of association between Acinetobacter and COVID infections cannot be ignored [12]. Acinetobacter baumannii was the main pathogen in the respiratory infections of COVID-19 patients (9.76%) and the rate was significantly higher than pre-pandemic group (3.49%, p < 0.002) as per a recent study by Karatas, et al. [28].
Our study had several limitations. Firstly, it was a retrospective observational study. Mortality was defined as all-cause mortality and not sepsis-related mortality because it was not always possible to determine the exact cause of death. However, because our outcome was death versus survival, we believe that infection was a contributing factor of mortality, if not the terminal event, in almost all cases.
Our study was conducted to evaluate risk factors and survival rates in patients infected with Acinetobacter infection. Our analysis suggests that the mortality was higher in males. The lesser the median time from hospitalization to diagnosis, higher was the mortality. Respiratory infections particularly ventilator associated pneumonia was the most common cause of A. baumannii infection. Association with COVID was an important observation as there was higher mortality when patients had COVID with A. baumannii (85.7%). High resistance to carbapenems was associated with high mortality (71.4%). Co-infection and/or secondary infection with other organisms especially Klebsiella was associated with higher mortality.
The optimal treatment for A. baumannii, especially nosocomial infections resulting from multiple resistant strains, remains to be established. We would like to emphasize that higher end antibiotics like carbapenems should be used judiciously, as resistance to these can have serious consequences as shown in our study, where colistin was the last resort.
It is thus a clinical imperative that well-designed protocol based management will guide clinicians on decisions regarding the current best practice. In view of rapidly evolving pandemic situation, appropriate preventive measures, hand hygiene, infection control and use of antibiotics judiciously is extremely crucial as this can, not only reduce mortality and morbidity but also help in decreasing prevalence of COVID-19 and A. baumannii infections.