Recent reports indicate that African Americans have higher mortality rates from SARS-CoV-2 coronavirus disease 19 (COVID-19) compared to Caucasians, with more marked differences in the Midwest region of the US. This study was performed to study differences in COVID-19 related mortality and hospital length of stay (LOS) between African Americans and Caucasians in Midwest setting, and identify factors associated with mortality and LOS.
Data were collected from the electronic health records (EHR) of patients admitted to hospitals in Midwest region of the US. EHR of 471 COVID-19 patients were reviewed.
Approximately 63% were African Americans and 34% Caucasians. One hundred sixteen variables were tested. There was no significant difference in hospital mortality between African Americans and Caucasians (OR 1, 95% CI 0.48-1.94). Older age, Chronic kidney disease, mental status change, mechanical ventilation, vasopressor support, high neutrophil count, elevated AST and ALT, high lung involvement severity score and elevated CRP were associated with mortality in a univariate analysis (P < 0.05). Multivariable modelling indicated that mechanical ventilation was the only factor that predicted mortality (OR 6, 95% CI: 2.94-12.48). The LOS did not differ in African Americans and Caucasians. The use of oxygen via high flow nasal cannula (Survival Estimate 1.6, 95% CI: 1.20-2.26), low estimated glomerular filtration rate (Survival Estimate 1.4, 95% CI: 1.05-1.82) and mechanical ventilation (Survival Estimate 3.5, 95% CI: 2.72-4.37) were predictors of LOS.
This study performed in Midwest setting in the US showed that race did not affect in-hospital mortality and LOS. Our analysis demonstrated new predictors of LOS.
COVID-19, Mortality, Outcomes, Length of hospital stay
Racial and ethnic disparities in health, health care utilization, morbidity and mortality have long been recognized [1]. In the U.S.A, African Americans have a shorter life expectancy than Caucasians with differences varying by state/region, with differences as high as 14.7 years in regions such as Washington, DC [2,3]. The racial disparity is not unique to the U.S. In South Africa, Blacks had the lowest rate of health insurance and least access to private health care [4]. In South America and Caribbean, Afro-descendants and indigenous population have lower socioeconomic status, impacting their health [5]. This disparity becomes even more apparent during pandemics. A recent review on race identified that African Americans had a higher case fatality rate than Caucasians during the 1918 Influenza pandemic [6]. A similar disparity was seen during the 2009 H1N1 pandemic, in which African Americans had higher in-hospital mortality rates as compared to Caucasians [7].
In the current coronavirus disease 2019 (COVID-19) pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), there is a concern that Blacks, including African Americans, are affected more than Caucasians, with proportionally more cases and increased mortality [8]. During this current pandemic, it was shown that US counties with a disproportionately higher black population accounted for 52% of COVID-19 cases and 58% of COVID-19 deaths [9]. In addition, there was a positive association between the percentage of African Americans living in a county and the percentage of COVID-19 confirmed cases, confirmed deaths and case-specific mortality [10]. It is believed that differences in the number of cases, hospitalization and mortality could be due to in adequate living conditions, increased comorbidities, increased exposure to SARS-CoV-2, poor access to health care and decreased awareness of the impact of COVID-19 on minorities [8,11]. These data have led a call for a National Commission to further explore racial/ethnic disparities and respond to the unique challenges of Black communities in COVID-19 pandemic [12]. Furthermore, a recent study from 9 US state health departments showed that cumulative mortality varies between African Americans and Caucasians in different states with worrisome outcome differences especially in states in Midwest [13]. These intriguing apparent regional differences of the impact of race on COVID-19 outcomes were hypothesized to be due to social, environmental and/or epidemiological factors. Therefore, this study was carried out, using data from COVID-19 patients hospitalized in one of the largest medical system in the Midwest U.S.A, with the aims of investigating outcome differences between African Americans and Caucasians and identifying factors associated with worse outcomes in different ethnic groups.
This was a retrospective study using Epic system of electronic health records (EHR, 1979-2020 Epic Systems Corporation, USA) of COVID-19 patients admitted to any of the seven Sisters of St. Mary (SSM) hospitals in Missouri including Saint Louis University Hospital, DePaul Hospital, St. Clare Hospital, St. Mary's Hospital, St. Joseph Hospital in Wentzville, St. Joseph Hospital in St. Charles and St. Joseph Hospital in Lake St. Louis. The SSM St. Louis region hospitals have a total bed number of 2274. The protocol was approved by the Saint Louis University Institutional Review Board and the study was performed in accordance with the relevant guidelines and regulations.
Data were collected from Epic system EHR of patients hospitalized for moderate or severe COVID-19. The hospitals included in the study were adult hospitals and admitted patients 18 years of age or older. To avoid bias from other serious co-infections, patients with other clear source of sepsis such as urinary tract infection, skin and soft tissue infection, bacteremia and other confirmed respiratory infections were excluded. Real-time reverse transcriptase polymerase chain reaction testing for SARS-CoV-2 RNA was performed on nasopharyngeal swabs under Emergency Use Authorization using the Abbott Realtime SARS-CoV-2 assay according to manufacturer's instructions (Abbott Molecular, Des Plaines, IL).
A list of patients who were tested for COVID-19 between February and December 2020 was obtained from a central microbiology laboratory that runs SARS-CoV-2 testing for all hospitals included in this study. To have a more complete list including patients transferred from other hospitals, we reviewed records of subjects who had ICD-10 diagnostic codes for confirmed COVID-19 (U07.1), ICD-10 codes for symptoms associated with COVID-19 (R05 for cough, R50.9 for fever and R06.02 for shortness of breath), pneumonia or lower respiratory tract infection (J12.89, J22), acute bronchitis (J20.8 or J40) and acute respiratory distress syndrome (J80). EHR of all subjects with at least one SARS-cov-2 RT PCR testing at the time of hospital admission or within 72 h after admission were selected for further review, data abstraction and entry into a standard data collection forms (DCF). Study staff who were certified to work with EHR and have completed Health Insurance Portability and Accountability (HIPPA) training carried out the abstraction according to detailed guidelines for data collection designed to avoid ambiguity in variable interpretation. The guidelines and DCF were revised in an iterative process to address additional nuances that were identified through pilot data abstraction of the medical records of 10 patients. Reproducibility of data abstraction was evaluated in the pilot study by comparing results of abstractions among abstractors.
Data were collected in Research Electronic Data Capture (RedCap) software (REDCap Software, version 6.0, Vanderbilt University) [14,15] using data collection forms that included demographics, initial clinical features, laboratory findings, available culture results, imaging findings, treatment received and outcomes. Lung involvement score was calculated as described in previous studies [16,17]. Briefly, each lung field is assessed separately with a score of 0 given for normal chest imaging; 1 if less than 25% of the lung field is involved; 2 if 25-50% is involved; 3 if 50-75% is involved; and 4 if more than 75% of lung is involved. The scores for each lung were summed to produce the final severity score.
Figure 1 shows a schematic of number of EHR reviewed and used for analysis. A total of 571 EHR were reviewed and 100 were excluded because they had other causes of sepsis. Of the 100 excluded patients, 51 had UTI, 27 had bacteremia, 42 has skin and soft tissue infection and 13 had a respiratory infection with a positive polymerase chain reaction (PCR) for adenovirus, Bordetella parapertussis, Bordetella pertussis, Chlamydia pneumonia, Coronavirus 229E, Coronavirus HKU1, Coronavirus NL63, Coronavirus OC43, Human Metapneumovirus, Human Rhinovirus/Enterovirus, Influenza A Non Subtyped, Influenza H1, Influenza A H3, Influenza A H1 2009, Influenza B, Mycoplasma Pneumoniae, Parainfluenza virus 1, Parainfluenza virus 2, Parainfluenza virus 3, Parainfluenza virus 4 and/or respiratory syncytial virus. Thirty-three of 100 excluded patients had more than one other causes of sepsis.
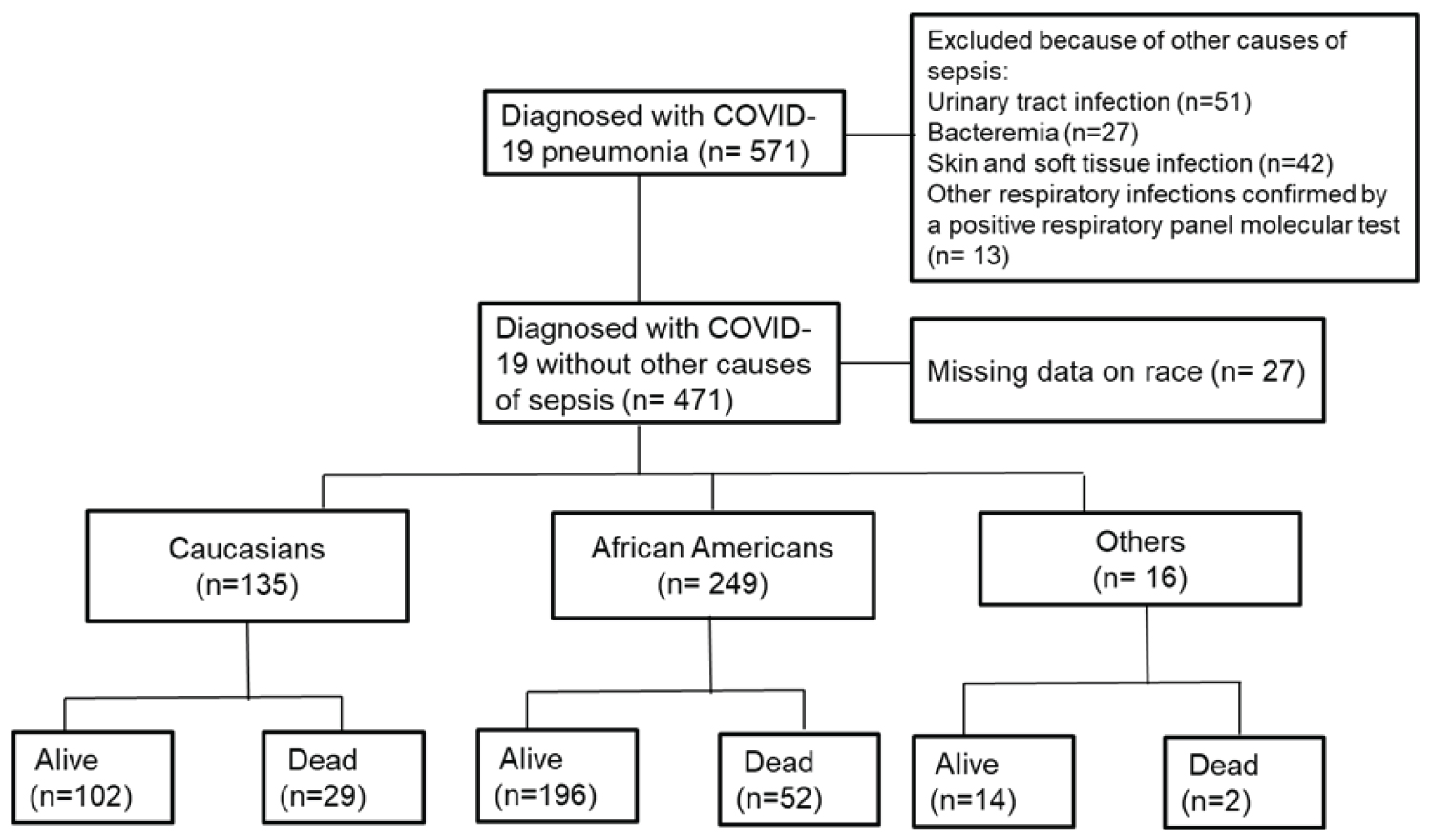 Figure 1: Schematic of number of hospitalized patients with COVID-19 pneumonia by race and hospital mortality.
View Figure 1
Figure 1: Schematic of number of hospitalized patients with COVID-19 pneumonia by race and hospital mortality.
View Figure 1
All-cause mortality was used as the primary outcome, and hospital length of stay (LOS) was used as the secondary outcome, defined as the number of days between hospital admission and discharge or death, whichever occurred first. For length of hospital stay, we evaluated patients up to day 14 post admission in multivariable modeling, with right censoring occurring at day 14. For patients who died prior to day 14, they were right censored at the worst outcome to account for competing risks in multivariable modeling [18]. One hundred and sixteen variables were tested for association with mortality in hospitalized African American and Caucasian COVID-19 patients separately.
Continuous data were reported as means with standard deviation (SD). Categorical variables were reported as frequencies with percentages. Interquartile range (IQR) was used in the analysis of duration of symptoms before hospitalization. Multivariable logistic regression was used to evaluate independent associations between race groups and all-cause mortality. Clinically relevant variables with bivariable associations (P < 0.25) with both race and mortality were included in the multivariate logistic regression model [19]. Independent associations between race groups and length of hospital stay were defined using a loglog distributed Accelerated Failure Time (AFT) model. Here, two models were created, one using variables with associations described in other studies or presumed clinical relevance based on previous reports, and the other using the same variables as included in the logistic regression model. P-values of < 0.05 were considered statistically significant in all analyses unless otherwise specified. R v4.0.3 (R Foundation for Statistical Computing, Vienna Austria) was used for all analyses.
The mean duration of symptoms before hospitalization was 5.3 days (IQR 2-7), 5.7 days (IQR 2.5-7), and 10 days (IQR 7-14) for African Americans, Caucasians and patients from other races, respectively. There was no significant difference in the duration of symptoms before hospitalization between African Americans and Caucasians (P = 0.55). Table 1 shows racial differences in baseline characteristics including demographics, comorbidities and home medication. Patients who were neither African American nor Caucasian were of younger age with a mean of 57.3 years. African Americans and Caucasians had a mean age of 63.3 and 67.2 years, respectively (P = 0.009). The average body mass index (BMI) in African Americans, Caucasians and others was 31.8, 29.5 and 29.1 kg/m2, respectively (P = 0.023). Chronic obstructive pulmonary disease (COPD) was more common in Caucasians (P = 0.036) whereas hypertension was more common in African Americans (P = 0.001). Home use of nonsteroidal anti-inflammatory drug (NSAID) and angiotensin receptor blocker (ARB) was more common in African Americans with P = 0.023 and 0.001, respectively. There were only 14 patients without any kind of health insurance, and there were no statistically significant differences in the number of patients with private insurance (26.9% vs. 19.3% vs. 12.5%, P = 0.15).
Table 1: Baseline characteristics of hospitalized COVID-19 patients. View Table 1
Clinical presentation, laboratory values and extent of lung involvement at presentation were similar between races, with the exception that fatigue, myalgia and elevated bilirubin were highest in 'other' races followed by African Americans and Caucasians (Table 2). In African Americans, Caucasians and other races hospitalized with COVID-19, fatigue was one of the presenting symptoms experienced by 29.7%, 19.3% and 43.8% of patients, respectively (P = 0.025). Myalgia was a presenting symptom in 12%, 3.7%, and 12.5% of African Americans, Caucasians and other races, respectively (P = 0.024). In African Americans, Caucasians and other races, elevated bilirubin was seen in 9.6%, 7.4% and 6.2% of cases, respectively (P = 0.005). C-reactive protein (CRP) was 10.2 ± 10.7 (Mean ± SD) in African Americans, 7.5 ± 8.3 in Caucasians, and 11.2 ± 7.4 in other races (P = 0.037).
Table 2: Clinical presentation, laboratory values and severity of illness at presentation in hospitalized COVID-19 patients. View Table 2
There were no significant differences among the different races in COVID-19 treatment or intravenous (IV) antibiotics received except in participation in the Remdesivir clinical trial [20] (Table 3). Among African Americans, Caucasians and other races, the proportion of patients who participated in the Remdesivir placebo-controlled trial was 4%, 0.7% and 12.5%, respectively (P = 0.023). Since this clinical trial was blinded, the intervention the patients received was not documented in the electronic health records. Vancomycin was the most commonly used empiric IV antibiotic followed by cefepime. Mortality in hospitalized COVID-19 African Americans, Caucasians and patients of other races was 20.9%, 21.5% and 12.5%, respectively (P = 0.225).
Table 3: COVID-19 treatment, length of hospitalization and outcomes by race. View Table 3
One hundred and sixteen variables were tested for association with mortality in hospitalized African American and Caucasian COVID-19 patients separately. Table 4 shows factors associated with mortality in at least one of the races. Eleven factors were significantly more common in both African American and Caucasian patients who died compared to those who were alive (P < 0.05). These factors include older age, chronic kidney disease (CKD), mental status change on presentation, high neutrophil count, elevated aspartate aminotransferase (AST), elevated alanine aminotransferase (ALT), elevated CRP, high lung involvement score on imaging studies, use of mechanical ventilation and vasopressor and empiric use of intravenous vancomycin and cefepime. Nine factors including history of COPD (P = 0.01), diabetes mellitus (P = 0.04), congestive heart failure (P = 0.05), statin use at home (P = 0.02), decreased lymphocyte counts (P < 0.001), increased bilirubin level (P = 0.001), high procalcitonin (P < 0.001), elevated D-dimer (P < 0.001) and inpatient steroid use (P = 0.005) were significantly more common only in African Americans who died. Six factors including the use of NSAID (P = 0.04) and beta blockers (P = 0.001) at home, cough as one of the presenting symptoms (P = 0.001), increased total WBC (P = 0.007), increased ferritin levels (P = 0.007) and use of bilevel positive airway pressure (BiPAP) (P = 0.004) were significantly more common only in Caucasians who died compared to those who survived. The use of hydroxychloroquine was more common in Caucasians who survived compared to those who died (P = 0.004).
Table 4: Factors associated with mortality in African Americans and Caucasians. View Table 4
Binary logistic regression analysis after adjusting for confounding variables (Figure 2) shows that African Americans and Caucasians did not significantly vary in all-cause in-hospital mortality (OR 1, 95% CI 0.48-1.94). Figure 2 also shows the impact of age, lung involvement score, CRP, elevated bilirubin, lymphopenia, history of NSAID use, COPD, myalgia as a presenting symptom, and intubation on mortality. Intubation/mechanical ventilation was the only factor that was associated with in-hospital mortality (OR 6, 95% CI: 2.94-12.48).
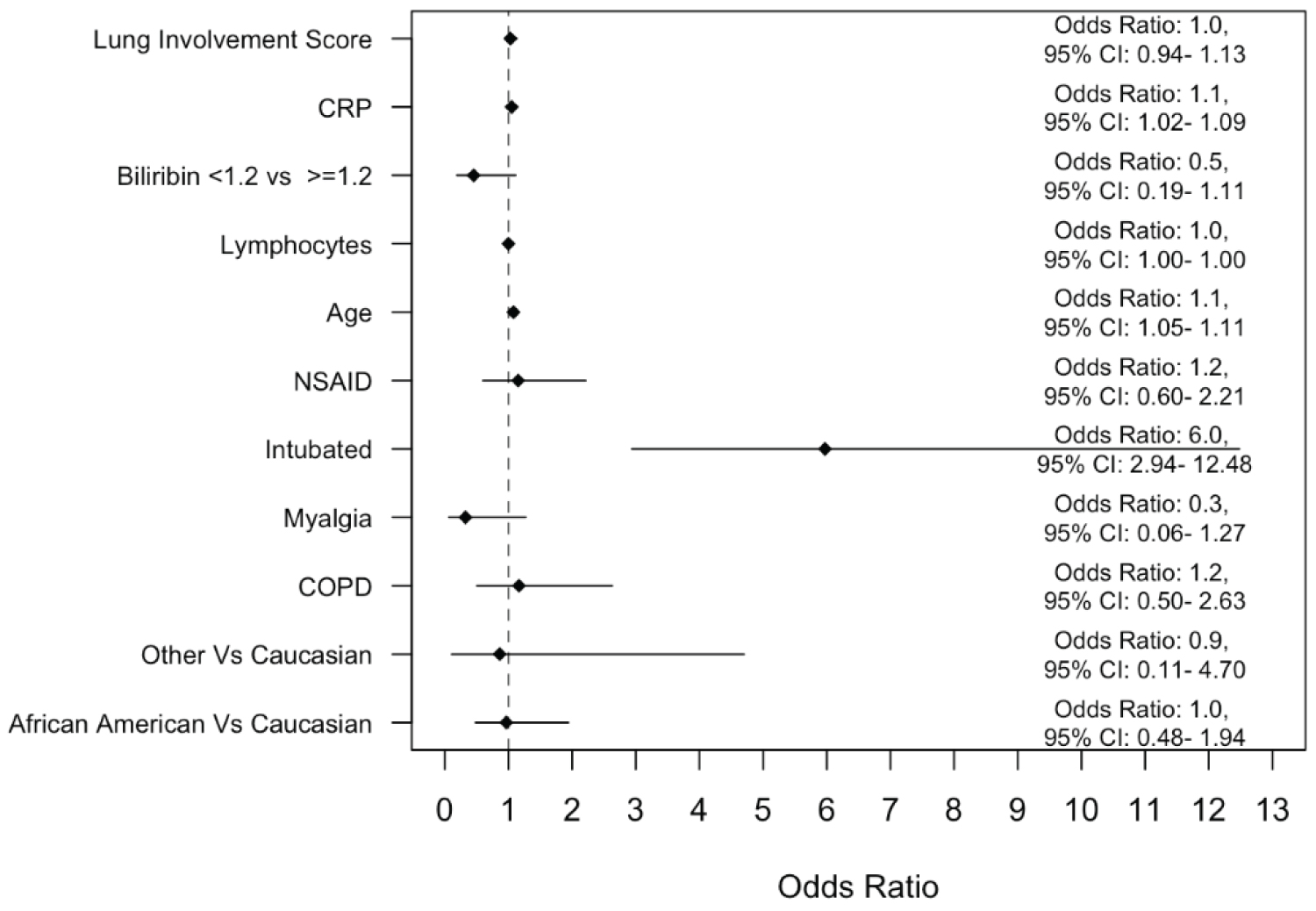 Figure 2: A binary logistic regression model to evaluate the impact of race on mortality after adjusting for several variables deemed clinically relevant.
Figure 2: A binary logistic regression model to evaluate the impact of race on mortality after adjusting for several variables deemed clinically relevant.
OR: Odds Ratio; CI: Confidence Interval.
View Figure 2
Figure 3 depicts multivariable modeling of time to hospital discharge using variables selected based on associations described in other studies and presumed clinical relevance or statistical significance [21]. In this model, time to hospital discharge was not significantly different between African Americans and Caucasians. Mechanical ventilation was a strong predictor of increased hospital LOS (Survival Estimate of 3.5, 95 CI: 2.72-4.37). The AFT for bilirubin was 0.7, indicating that patients with bilirubin < 1.2 had a 30% decrease in the hospital LOS compared to those with bilirubin > 1.2. The use of HFNC (Survival Estimate of 1.6, 95% CI: 1.20-2.26), low eGFR (Survival Estimate of 1.4, 95% CI: 1.05-1.82)) and hemodialysis (Survival Estimate of 1.5, 95% CI: 1.07-2.14) were associated with increased hospital LOS.
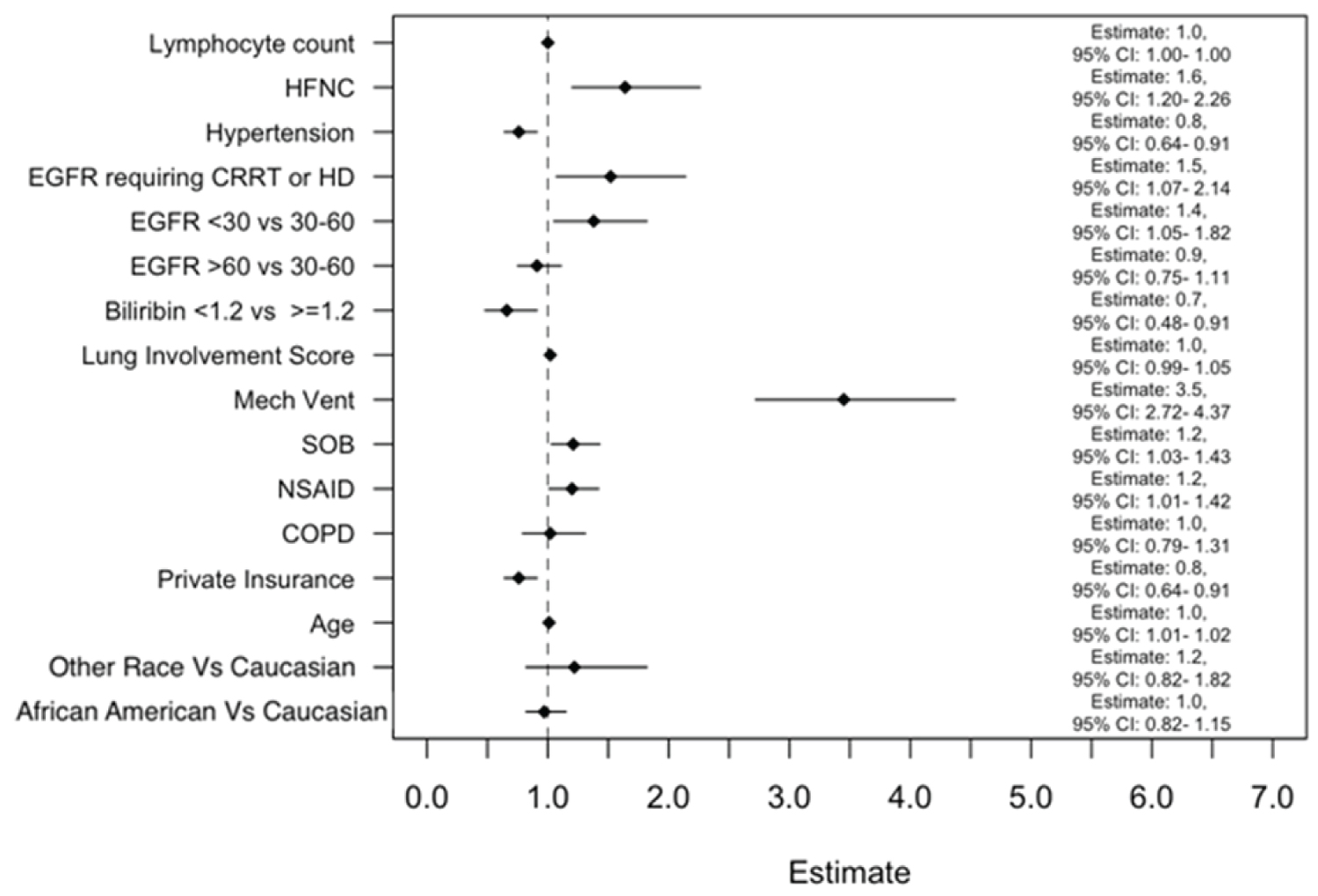 Figure 3: Multivariable modeling of time to hospital discharge (days) using variables that are selected based on clinical relevance from reports in other studies or presumed clinical and statistical association (P < 0.05).
View Figure 3
Figure 3: Multivariable modeling of time to hospital discharge (days) using variables that are selected based on clinical relevance from reports in other studies or presumed clinical and statistical association (P < 0.05).
View Figure 3
The COVID-19 pandemic has clearly delineated health disparity among African Americans and Caucasians. In a cross-sectional study of adults tested for COVID-19, the positivity and hospitalization rates were associated with race and poverty [22]. Hospitalization rates in African Americans are reportedly 1.5 to 4 times higher than Caucasians [23]. Although the reasons for this difference are not well defined, it is believed that health care access and exposure risk could be contributing factors. Minorities including African Americans were more likely to be employed in essential industries with little protection and more exposure at the height of the COVID-19 pandemic [11]. Situations that may lead to loss of social distancing has been shown to cause easy spread of SARS-CoV-2 in communities [24]. With increased rates of hospitalization, it would not be surprising to assume that African Americans have higher rates of COVID-19 associated mortality. As expected, a recent systematic review found that mortality from COVID-19 is higher in African Americans [23,25]. However, these findings are not universal as other studies have shown no difference in COVID-19 associated mortality among the different races [26]. One explanation for these conflicting results on the effect of race on mortality could be geographic location [13,25]. For instance, Midwestern states of US including Missouri appear to have the largest differences in mortality between African Americans and Caucasians [13,25]. Interestingly, our study, in a hospital setting with a large number of African Americans, showed that there was no significant difference in the inpatient mortality between African American and Caucasian COVID-19 patients.
About 34% of our patients were Caucasians and 63% were African Americans although African Americans comprise only 12% of Missouri's population. This discrepancy could either be due to preferential utilization of SSM health services by African Americans or increased severity of COVID-19 and hospitalization in African Americans as described previously [22]. The findings from a recent systematic review further support the idea that African Americans experience disproportionately higher rates of hospitalization compared with non-Hispanic Whites [27].
In the US, where race is one of the major factors affecting the epidemiology of COVID-19, our univariate analysis showed that 23 variables have significant association with mortality in African Americans (P < 0.05). Eleven of these variables also showed association with mortality in Caucasians. These variables included advanced age, elevated CRP and comorbidities reported previously as independent risk factors [28-30]. A recent meta-analysis comprised mostly of research papers from outside the US suggested that BMI was associated with mortality. However, in our study, BMI was not found to be a risk factor for in-hospital mortality. A similar finding showing lack of association between BMI and COVID-19 mortality was obtained in other US studies [31]. Our multivariate logistic regression showed that only mechanical ventilation was significantly associated with mortality. Similar to the results obtained in studies from Georgia, African Americans and Caucasians in our study did not differ in the rates of mechanical ventilation [32]. A meta-analysis of 69 studies, 23 from North America, showed that almost half of patients with COVID-19 who require mechanical ventilation died, indicating that mechanical ventilation is a predictor of in-hospital mortality [33].
In this study, African Americans and Caucasians did not significantly differ in LOS. The average hospital LOS was 9.7 days in African Americans and 9.2 days in Caucasians. The average hospital LOS in our study are much shorter than average LOS of 14 days reported from Ohio [34] but longer than the 4 days of average LOS reported from New York [35]. The reason for this difference in the average LOS is not clear but severity of illness as evidenced by proportion of patients who required mechanical ventilation may have contributed. In our study, 21% of patients required mechanical ventilation whereas in the studies from Ohio and New York, 43% [34] and 4% [35] of patients required mechanical ventilation, respectively. To further confirm the association of statistically selected variables with LOS as an indicator of severity of illness, we used Accelerated Failure Time model and assigned death the maximum LOS. Mechanical ventilation was found be the major factor associated with LOS, followed by use of high flow nasal cannula oxygen and low eGFR.
Noninvasive ventilation methods including HFNC, CPAP and BiPAP have helped delay the need for mechanical ventilation. A systematic review suggested that HFNC compared to conventional oxygen therapy may reduce the need for invasive ventilation [36]. CPAP use has been associated with decreased risk of death in COVID-19 patients hospitalized for < 7 days [37]. In our study, HFNC, CPAP and BiPAP did not have association with mortality.
We used a multicenter hospital database to address a key question of the effects of African American race on severity, hospital LOS and mortality in the US Midwest. However, this study has limitations: i) Analysis may be affected by variability in decision to transfer patients to ICU and on time of discharge among the different hospitals, ii) It does not assess severity and mortality after hospital discharge or during readmissions and iii) It does not assess severity and mortality in patients who were never admitted to hospital.
In conclusion, this study shows that in the Midwest hospital setting in Missouri, African Americans and Caucasians do not significantly differ in hospital LOS, length of ICU stay and mortality. Mechanical ventilation, HFNC and low eGFR are associated with longer hospital stay but only mechanical ventilation is associated with mortality.
The work was approved by the Saint Louis University institutional review board (IRB) and SSM health administrative review board. The approved protocol includes documents of exemption from consent and use of data (i.e., data analysis and publication) without identifiable private health information. The study was performed in accordance with the relevant guidelines and regulations.
This is a retrospective study and informed consent waiver was obtained from the Saint Louis University IRB.
Data and materials presented in this study are collected and analyzed after obtaining approval from the Saint Louis University IRB. The datasets analyzed during the current study are not publicly available due to Institutional Review Board (IRB) requirements, but portions of these data will be available from the corresponding author on reasonable request and IRB approval.
All authors do not have any conflict of interest.
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
All authors contributed to writing the manuscript. GA, AK, EC, SF and DF were involved in the research design. GA, AK, EC, BB, QW, GW, MS, JZ and RC were involved in data collection. TW and GA were involved in data analysis. All authors provided comments to the various drafts and approved the final version.
The datasets analyzed during the current study are not publicly available due to Institutional Review Board (IRB) requirements, but portions of these data will be available from the corresponding author on reasonable request and IRB approval.