Similar to that of bacterial infection, the performance of diagnostic tests for endemic fungal pneumonia and opportunistic fungal pneumonia are uncertain.
We undertook a literature search to assess the accuracy of diagnostic tests for pneumonia, identified through a search of MEDLINE-indexed journals. Sensitivity and specificity of diagnostic tests for pneumonia were calculated with respect to various reference standards.
A combination of diagnostic testing is adequate to rule out most endemic fungal pneumonia, Cryptococcus, and Candida. Testing is inadequate to exclude Pneumocystis, Aspergillus, and Mucorales, and empiric treatment should be considered when there is clinical suspicion.
The accuracy of any single diagnostic test for either endemic fungal pneumonia or invasive fungal pneumonia is generally inadequate to rule out a pathogen. Multiple diagnostic methods are often needed to confidently establish a microbiologic diagnosis for most cases of invasive fungal infection.
Pneumonia, Diagnosis, Accuracy
Clinicians often face uncertainty with respect to the accuracy of diagnostic laboratory tests for common endemic fungi present in various regions of the world. Additionally, with the growing use of immunosuppressive medications and chemotherapy, clinicians must be familiar with the laboratory tests needed to diagnose disease caused by opportunistic invasive fungal infection (IFI).
In Part 2 of this review, we will assess the literature and provide the accuracy of diagnostic tests for infectious pneumonia caused by fungal pathogens.
Coccidioides immitis is a large dimorphic fungus, endemic to the southwestern United States and parts of South America, transmitted through inhalation of fungal spores.
The sensitivity of respiratory secretions culture for Coccidioides has been reported at 80% [1] of PCR-positive cases, with a specificity of 100% [1]. The sensitivity of culture for cases that are diagnosed clinically or serologically is uncertain, as most diagnoses are made by culture itself.
BALF cytology sensitivity has been reported at 35% [2] of culture-positive cases, and is not known to provide additional yield to culture. Transbronchial biopsy sensitivity for this population has been reported at 100% [2], but similarly may not provide additional information while increasing the procedural risk.
Various antigens have been used to diagnose Coccidioides, including the cell wall polysaccharide galactomannan (GM). The Coccidioides GM EIA UAT sensitivity has been reported at 83% [3] of culture-positive cases, but reduces to 50% [4] and 68% [3] of cases diagnosed by either culture or ID serology, with a specificity of 98% [3]. The UAT cross-reacts with other endemic fungi such as Histoplasma and Blastomyces, limiting its value [3]. The UAT may detect Coccidioides in serologically-negative cases by ELISA, although it is not certain if this provides additional sensitivity to the serologic combination of ELISA, CF, and ID [3]. Antigenemia sensitivity among culture-positive or serologically detected cases has been reported at 73% [4], adding up to an additional 17% [4] sensitivity to that of the urine antigen alone. Conversely, the UAT adds little sensitivity to the serum antigen detection, and is thus not recommended.
Serology may complement IS or BALF culture. Among culture-positive or histopathology-proven cases, ELISA IgG and IgM serology sensitivity has been reported at 67% [5], but paradoxically in cases detected by either culture, histopathology, CF, or ID has been reported at 83% [6] and 84% [7]. The specificity has been reported at 90% [6] and 95% [7], but titers may remain elevated for months. CF sensitivity has been reported at 58% [8], 63% [3], and 67% [5] of cases diagnosed by either culture, serology, or histopathology, and is not certain to add cases to those detected by ELISA [9]. ID sensitivity has been reported at 53% [5] and 54% [3] of cases diagnosed by either culture, serology, or histopathology. The combination of ELISA, CF, and ID can increase sensitivity to as high as 84% [5] in immunocompromised patients, and as high as 95% [5] in those without an immunocompromising condition. ELISA, CF, and ID are all commercially available, and as the combination of serologic methods can increase sensitivity, at a minimum the ELISA and ID methods should be used concurrently.
Among culture-positive cases, the sensitivity of PCR in respiratory secretions has been reported at 100% [1], with a specificity of 98% to 100% [1]. PCR of fresh tissue sensitivity has been reported at 93% [1] and 94% [10] of culture-positive cases, with a specificity of 98% [10] and 100% [1]. The sensitivity reduces in paraffin-embedded tissue, however, to 73% [1]. The role of PCR is uncertain, but should be considered if culture and serology are negative with a high clinical suspicion.
Blastomyces dermatitidis is an endemic dimorphic fungus of the midwestern and eastern United States, Africa, and India, transmitted through inhalation of spores.
BALF culture sensitivity has been reported at 67% [11] of cases with a positive culture by any means. This value is likely overestimated due to clinical under diagnoses by alternative methods such as serology or antigen detection. For example, among pathologically confirmed cases, culture sensitivity has been reported at only 57% [12], 66% [13], and 67% [13]. IS culture is less invasive than obtaining BALF, and may even have a higher sensitivity than BALF culture [11]. Colonization is not known to occur and thus any positive culture should be considered infectious with a specificity approaching 100% [11].
Transbronchial biopsy is diagnostic of Blastomyces in only 22% [11] of culture-positive cases, but can add up to 20% additional yield [14] to culture alone, although the procedure carries an increased risk over bronchoalveolar lavage.
The Blastomyces UAT has been detected in 90% [15] and 93% [16] of culture-positive or histologically-proven cases, with a specificity of 79% [16], since cross-reaction may occur with other endemic fungi such as Histoplasma, Cryptococcus, and Paracoccidioidomycosis. The specificity has been reported as high as 99% [15] in healthy individuals without alternative fungal infection. Antigen detection in either culture-positive or histology-proven pulmonary disease can be found in urine, serum, and BALF with reported sensitivities of 83% [17] and 100% [16] for urine, 64% [17] for serum, and 63% [17] for BALF. Skin testing is infrequently performed, although among clinically diagnosed cases sensitivity has been reported at 73% [18]. Similarly, lymphocyte transformation assays are infrequently used because the sensitivity has been reported at only 68% [19] of cases positive by either serology, culture, or skin testing. Theses assays do not appear to detect cases which are not already found by more traditional methods.
Serologic methods have been utilized to detect the A-antigen and surface protein Blastomyces adhesion (BAD-1). Among culture-positive cases of blastomycosis, EIA sensitivity has been reported at 77% [18], 80% [19], 83% [20], 88% [21,22]. The sensitivity has been reported higher in clinically suspected disease at 97% [18], but this likely overestimates the true sensitivity, which is presumably closer to the sensitivity estimated from culture-positive cases. Its specificity has been reported at 92% [18] and 94% [21], being reduced due to cross reaction with other endemic fungi [23]. ID appears to be less sensitive than EIA, and among clinically diagnosed cases has been reported at only 50% [18]. Similarly, among culture-positive cases, the sensitivity of ID has been reported at only 15% [21], 28% [18], 40% [11], and 65% [19], with a specificity approaching 100% [18]. CF sensitivity has been reported at only 15% [18] of clinically diagnosed cases, while among culture-positive cases the sensitivity has been reported as low as 9% [18], 16% [24], 40% [19], and 43% [22], although with specificity approaching 100% [18]. Serology by EIA, CF, and ID are all commercially available and should be used together to maximize diagnostic yield.
Among culture-positive cases, the sensitivity of Blastomyces PCR in tissue and respiratory secretions has been reported at 83% [25] and 86% [26], respectively, with a specificity reported at 93% [26] and 100% [25]. PCR is commercially available, although it is unclear if it provides additional benefit over the combination of culture and serology. Although promising as a complementary diagnostic test to serology, the role of PCR as a diagnostic tool is uncertain.
Histoplasma capsulatum is a small dimorphic fungus, most commonly found in midwestern United States, Central America, South America, southern Africa, and Southeast Asia, transmitted by inhalation of spores.
The sensitivity of respiratory culture varies by the duration of symptoms, and increases from acute to chronic stage of disease. The sensitivity is also higher in cavitary disease than in non-cavitary disease [27]. Culture sensitivity in the subacute phase of infection has been reported at 54% [28], 61% [29] and 78% [30] of UAT-positive cases, 83% [31] of clinically diagnosed cases, and 89% [29] of cases positive by histopathology, culture, or serology. It may also be positive in instances of a negative urine antigen, and is thus complementary. Fungal growth may require up to four weeks, however, limiting its usefulness in the acute or subacute phases of infection. Nevertheless, while BALF culture is frequently negative in the acute phase [27,28], its sensitivity has been reported to increase to 67% [28] in the chronic phase of cases diagnosed by either culture or serology. Sputum culture sensitivity may be slightly better than BALF in the acute phase and has been reported at 18% [27] of cases positive by either culture, cytology, histopathology, CF, or ID.
BALF cytology sensitivity has been reported at 55% [31] and 70% [32] of clinically diagnosed cases. The sensitivity reduces, however, to 19% [27] of cases detected by either culture, cytology, histopathology, CF, or ID. Tissue pathology is diagnostic in 67% [28] of culture-positive or serologically-diagnosed cases, but like culture is frequently negative in the acute phase [28].
The pooled sensitivity of antigenuria and antigenemia detection by ELISA and radioimmunoassay (RIA) methods among cases detected by either culture, serology, or PCR have been reported at 79% [33] and 82% [33], respectively, with serum and urine specificity of 97% [33] and 99% [33]. The Histoplasma UAT sensitivity varies by the stage of disease and can be reduced to as low as 39% [28] in the subacute phase before rising to over 80% [28] in cases of chronic infection found to be positive by either culture, cytology, histopathology, CF, or ID. BALF Histoplasma antigen sensitivity among culture-positive cases has been reported at 50% [30], 70% [32], and 84% [34], but has poor specificity due to cross-reactivity with Blastomyces.
Serology can be used to detect the Histoplasma H and M antigens. ID serology has been reported positive in 17% [35] of cases diagnosed either clinically or by culture, and 25% [31], 55% [35], and 94% [36] of those with a confirmatory positive antigen, serology, or culture. ID sensitivity has been reported at 87% [27] of CF-positive cases, and appears to add minimal additional yield to CF alone [27], with a specificity of 96% [27] and 100% [31]. Among clinically diagnosed cases of histoplasmosis, CF sensitivity has been reported at 64% [35] and 91% [37], and among those diagnosed by either by culture or a positive antigen has been reported at 73% [38] and 87% [36]. Specificity is also poor due to cross-reaction with other endemic fungi [27], and titers may persist for months to years. CF and ID methods may be complementary. For example, the combination of CF and ID increases sensitivity to a reported 82% [28] of cases detected by either culture, cytology, or histopathology, and to 96% [27] of cases detected by any method of culture, cytology, histopathology, CF, or ID. Both CF and ID serology are widely commercially available. Although ID is not certain to provide additional sensitivity to CF, it may yield a result more rapidly than CF. Negative serology does not rule out infection, however, and PCR or tissue diagnosis should be considered if there is persistent clinical suspicion.
The sensitivity of PCR in respiratory secretions has been reported at 73% [26] of culture-positive cases, with a specificity of 100% [26]. The addition of culture to PCR can increase the sensitivity by up to an additional 30% [31], so multiple diagnostic modalities should be considered. Serum PCR sensitivity has been reported at 60% [31] and 77% [39] of culture-positive cases, with a specificity of 90% [31], while the sensitivity of bone marrow PCR in disseminated disease has been reported at 92% [40]. Histoplasma PCR is commercially available although uncommonly utilized. Although the diagnostic accuracy in BALF is uncertain, it should be considered whenever serology is negative.
LAMP is an alternative to PCR and among culture-positive cases the sensitivity has been reported in bone marrow and urine at 54% [40] and 67% [41], respectively. LAMP however, is neither widely commercially available nor well-studied in BALF specimens (Figure 1).
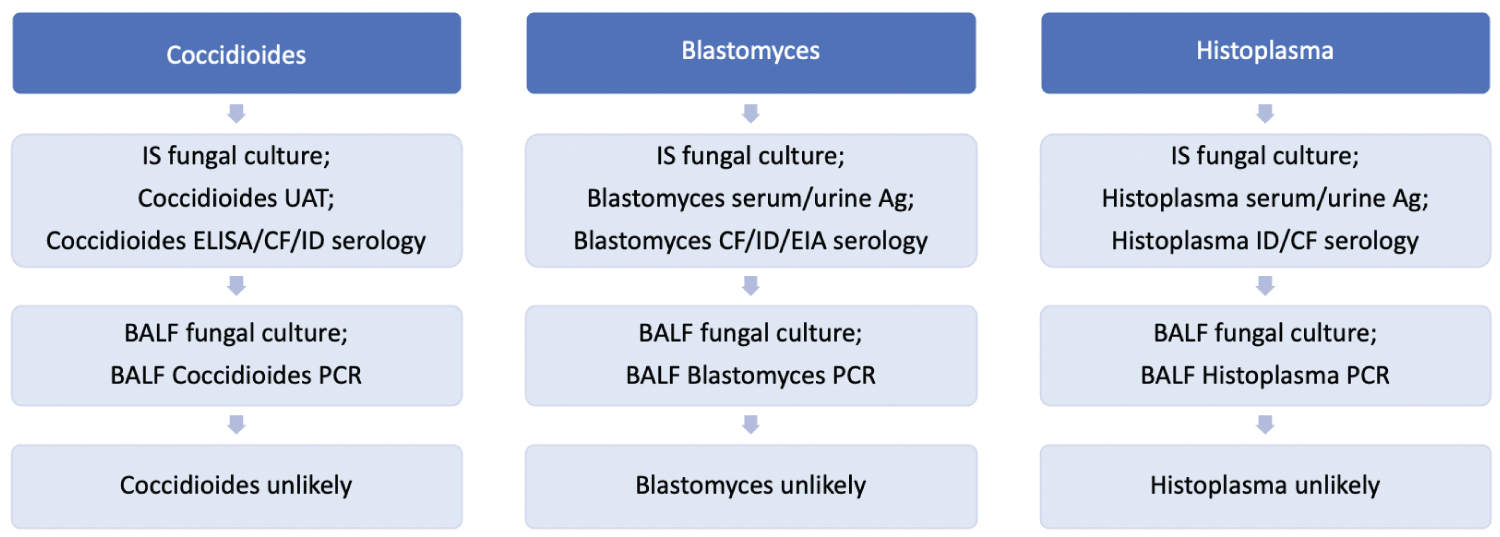 Figure 1: Endemic fungal pneumonia ("*" Indicates that the test is not widely commercially available).
View Figure 1
Figure 1: Endemic fungal pneumonia ("*" Indicates that the test is not widely commercially available).
View Figure 1
The European Organization of the Research and Treatment of Cancer/Mycoses Study Group (EORTC) criteria are often used to support the diagnosis of invasive fungal infection (IFI). Proven invasive disease is defined by evidence of tissue damage with a positive culture. Probable disease is defined by the presence of underlying host factors, clinical criteria, and mycological criteria.
Cryptococcus species are encapsulated yeast that can lead to pneumonia following inhalation of fungal spores. Risk factors for infection include HIV infection, solid organ or bone marrow transplantation, diabetes mellitus, chronic glucocorticoid use, malignancy, and other immunosuppressive medication use [42].
The pooled sensitivity of BALF culture has been reported at 60% [43] of histologically-proven or cytology-proven cases. Of these histologically proven cases, tissue culture has been reported positive in a wide range at 0% [44] and 71% [45]. BALF culture sensitivity has been reported at 93% [46] of cases in which the serum Cryptococcus antigen (CryAg) is detected, but is reduced to 63% [44] when compared to cases found to be positive by either the CryAg or biopsy. Colonization with Cryptococcus is common in the absence of pneumonia, and thus specificity for infection is low.
The sensitivity of cytology has been reported at only 47% [47] of culture-positive cases, but cytologic examination of BALF may be positive in up to 20% [44] of culture-negative clinically diagnosed cases. Transbronchial biopsies have been reported positive in 75% [44] of culture-positive cases, but can also detect up to 20% [44] of culture-negative infections. Therefore, tissue sampling may be considered concurrently with BALF culture. Any added benefit of transbronchial biopsies over the combination of culture, CryAg, and PCR, however, is uncertain.
BALF CryAg can be detected using latex agglutination (LAT), EIA, or lateral flow assay (LFA) methods. The sensitivity of BALF CryAg in clinically diagnosed cases has been reported at 93% [48]. Among culture-positive cases, BALF CryAg sensitivity has been reported at 71% [49], 80% [50], 83% [51], and 100% [52,53], with a specificity of 97% [54] and 99% [49,50], illustrating its ability to detect cases missed by culture alone. The CryAg can also be detected in tissue with a reported sensitivity of 100% [54] of cases positive by either histopathology or culture. Serum CryAg sensitivity is similar to that of BALF and has been reported at 56% [55], 74% [51], 75% [48], 93% [52], and 96% [45] of cases positive by either histopathology or culture, with a specificity of 99% [51]. Serum CryAg should be considered concurrently with IS or BALF culture. The pooled urine CryAg sensitivity is similar to serum at 85% [56], but infrequently utilized and not generally recommended as it is uncertain whether it adds additional cases to serum alone.
Serology is neither commercially available nor well-studied. IF sensitivity has been reported at 20% [57] of culture-positive cases, but with such low sensitivity lacks a role in diagnosis.
BALF PCR sensitivity has been reported at 80% [58] and 100% [59,60] of culture-positive cases. It is also uncertain whether PCR provides additional sensitivity to that of BALF culture and CryAg alone. All the same, the addition of PCR may be considered if there is high clinical suspicion.
Aspergillus species are narrow septate hyphae molds that lead to pneumonia in patients with immunocompromising conditions. Invasive aspergillosis (IA) is a form of severe opportunistic pneumonia distinct from chronic necrotizing pulmonary aspergillosis (CPA), aspergilloma, or allergic bronchopulmonary aspergillosis (ABPA). Risk factors for invasive aspergillosis include neutropenia, hematologic malignancy, allogeneic stem cell transplant, solid organ transplantation, prolonged glucocorticoid use, immunosuppressive medication use, and inherited immunodeficiency syndromes [61].
Although Aspergillus grows rapidly, the sensitivity of BALF culture for IA is poor, and has been reported in 10% [62], 25% [63], 30% [64], 33% [65], 36% [66], 50% [67], and 56% [68] of cases meeting proven or probable EORTC criteria. Among patients with histopathologically-proven IA, the sensitivity remains low at 35% [69], 37% [69], 40% [70] and 64% [71]. The yield of sputum may be up to twice that of BALF owing to the presence of Aspergillus in tracheal secretions [62,64], and procuring culture by IS is less invasive than by bronchoscopy. Specificity of culture is poor due to frequent colonization in non-infected individuals, and a diagnosis of IA should only be made with support of clinical, radiologic, and serologic findings [69,71].
Aspergillus ELISA IgA sensitivity has been reported at 17% [72] and 29% [73] of cases meeting EORTC criteria for IA. ELISA IgG sensitivity has been reported at 50% [74], 80% [75], 83% [72], and 74% to 88% [76] of cases meeting EORTC criteria for CPA, although specificity appears to be low, consistent with that of invasive disease [77]. ID sensitivity has been reported at 38% [78], 70% [79], and 88% [79] of histopathologic-proven cases, and 56% [80] of clinically diagnosed cases. Serology can provide additional cases to GM alone, but due to the long processing time and an inability to distinguish between invasive aspergillosis, aspergilloma, or allergic bronchopulmonary aspergillosis (ABPA), serology is generally not recommended.
Serum galactomannan (GM), a cell wall component of Aspergillus, has a pooled sensitivity reported at 71% [81] of cases with proven EORTC criteria and 61% [81] of cases with either proven or probable EORTC criteria, with a specificity of 89% [81] for proven IA and 93% [81] for proven or probable IA. The sensitivity is reduced in solid-organ transplant recipients [81], but high in immunocompromised patients with a pooled sensitivity reported at 78% [82] of those cases meeting EORTC criteria, and a specificity of 85% [82]. The sensitivity is slightly lower and specificity slightly higher when using an ODI threshold of 1 instead of 0.5 [82], but nevertheless is only a modestly sensitive test, so a negative culture or GM should not preclude further diagnostic testing.
The pooled sensitivity of BALF GM exceeds that of serum, having been reported at 82% [83], 87% [83], and 90% [84] of cases meeting proven or probable EORTC criteria, with a specificity of 89% [83] and 92% [85]. The diagnostic accuracy is similar when using ODI cutoff values of either 0.5 or 1.0. The specificity might be reduced for those on beta-lactam antibiotics, but this controversy has not been fully resolved [85]. The pooled sensitivity for cases with proven EORTC criteria is even better, having been reported positive in 94% [85] and 100% [48] of cases, with a specificity of 72% [84]. Although the sensitivity of BALF appears to exceed that of serum by a reported 10% [86] and 40% [87], either source may be independently positive and thus in cases with negative serum GM, BALF GM should be obtained [46].
Serum β-D-glucan (BDG) pooled sensitivity has been reported at 77% [88] with a specificity of 83% [88]. When both BDG and GM are positive, the specificity improves to 98% [89,90] and a diagnosis may confidently be made. The sensitivity, however, is reduced to 49% [90] and 55% [89]. Thus, BDG and serum GM are excellent initial studies, and they may be diagnostic of IA if both are positive when used in conjunction with clinical findings.
BALF Aspergillus PCR pooled sensitivity has been reported at 90% [91] of cases meeting proven or possible EORTC criteria, with a specificity of 96% [91]. The addition of BALF GM to PCR has been reported to improve the sensitivity to 84% [85] and 97% [91], with a specificity of 98% [91]. Serum PCR pooled sensitivity has been reported at 79% [92], 84% [93], and 88% [94] of cases meeting proven or possible EORTC criteria, with a specificity of 75% [93] and 80% [92]. As is the case with BALF, the addition of GM to serum PCR has been reported to improve the sensitivity up to 99% [93]. PCR may also improve the detection of Aspergillus, but should be used in conjunction with culture and fungal cell wall component testing.
Pneumocystis jirovecii is an ascomycetous fungus that can lead to pneumonia in immunocompromised patients. Risk factors for pneumonia (PJP) include lymphopenia, the use of immunosuppressive medications excluding mycophenolate, chronic glucocorticoid use, HIV infection, and solid organ transplantation [61].
Culture is not performed as the fungus fails to routinely grow on standard medium, although culture can be performed successfully under special conditions [95]. The sensitivity is therefore uncertain. Instead, cytology, DFA, and PCR are the means most commonly used to diagnose PJP. Grocott’s methanamine silver stain (GMS) is the preferred cytologic method with a sensitivity having been reported at 77% [96] of cases with two confirmatory stains, with a specificity of 99% [96].
BALF direct fluorescent antibody (DFA) is an alternative to conventional staining methods, with a sensitivity having been reported at 90% [97] and 99% [98] of cases positive by conventional stains, with a specificity of 86% [97] and 94% [99]. Although DFA is commonly used as an initial diagnostic test, its sensitivity is poor when compared against PCR. Among PCR-positive specimens, the sensitivity has been reported at only 33% [100], 47% [101] and 93% [102], while IF sensitivity has been reported at 71% [103]. IS and expectorated sputum DFA can be obtained in place of BALF as an initial diagnostic test, with sensitivity in clinically diagnosed cases having been reported at 48% [99] and 55% [104], respectively.
BDG is commonly elevated in Pneumocystis infection. Specificity is poor, however, due to cross reactions with other opportunistic fungi as previously discussed. The pooled sensitivity of serum BDG has been reported at 95% [105] and 96% [88] of patients positive by either DFA, PCR, or histopathology, with a specificity of 84% [88] and 86% [105]. BDG can be detected in BALF, but is less sensitive than serum BDG and is not certain to provide additional sensitivity [106].
BALF Pneumocystis PCR sensitivity has been reported at 83% [107] cytology-proven cases, and pooled 98% [108] of fluorescent-positive cases, with a specificity of 91% [108]. The sensitivity in clinically suspected cases, interestingly, reduces to approximately 44% [108]. This may underestimate the true sensitivity as a result of misdiagnosis of alternative processes such as CMV, while the pooled 98% figure may be attributed to an overestimation of cases undiagnosed by either PCR or DFA. The positive correlation between BDG and PCR positivity, however, indicates that the sensitivity of PCR is likely very high. IS sensitivity may even exceed that of BALF [103]. A combination of DFA, BDG, and PCR should be considered in diagnosis of PJP, however none of these has adequate sensitivity to rule out infection, so empiric treatment should be considered when there is high clinical suspicion.
Candida species are ubiquitous environmental yeasts that often colonize airways, but can lead to pneumonia in a patient with an immunocompromising condition. Risk factors for infection include neutropenia, hematologic malignancy, allogeneic stem cell transplant, solid organ transplant recipient, prolonged corticosteroids, T-cell suppressing medication use, inherited severe immunodeficiency, and acute graft-versus-host disease [61].
BALF culture sensitivity among histopathologically-confirmed cases of invasive Candida pneumonia has been reported at 70% [109], 83% [110], 90% [111], and 100% [112]. Despite its excellent sensitivity, specificity of culture for invasive disease is poor, reported at 0% [24], 20% [112], and 57% [113], owing to a high frequency of non-pathologic colonization. The accuracy of culture by IS appears to be similar to that of BALF [113], but IS sample collection is less invasive. IS or BALF culture is recommended with the understanding that a positive culture should not be considered diagnostic of invasive pneumonia without pathologic evidence of tissue invasion.
Serum or BALF BDG detection supports a diagnosis of invasive fungal disease. The pooled sensitivity of serum BDG for invasive candidiasis, including infection outside of the respiratory tract, has been reported at 81% [88] with a specificity of 81% [88]. BALF and IS BDG sensitivity among clinically suspected cases of Candida pneumonia have been reported at 89% [114] and 67% [114], respectively, with a specificity of 86% [114] and 82% [114]. As previously noted, BDG can be elevated in other invasive fungal diseases such as IA and PJP.
BALF PCR sensitivity for Candida pneumonia has been reported at 89% [115] of culture-positive cases. The sensitivity of PCR of cases confirmed by histopathology, however, is unknown. Likewise, the specificity of PCR is also unknown but should be consistent with the low specificity of culture in general. Among clinically diagnosed invasive candidiasis (IC) the pooled sensitivity of serum PCR has been reported at 95% [116] with a specificity of 92% [116]. While PCR positivity may supplement clinical suspicion, it has insufficient specificity to permit a trustworthy diagnosis of invasive pneumonia without pathologic confirmation.
Mucorales are an opportunistic group of fungi that can lead to pneumonia through the release of inhaled spores. Risk factors for pneumonia include chronic glucocorticoid use, diabetes mellitus, neutropenia, hematologic malignancy, hematopoietic cell transplantation, solid organ transplantation, and AIDS [61].
BALF culture sensitivity has been reported at 45% [117] and 66% [113] of those with diagnostic histopathology, and pooled at 43% [118] of those with proven or possible EORTC criteria, with a specificity that approaches 100% [113]. Although the sensitivity of culture has been reported at only 20% [118] and 42% [116] of PCR-positive cases, it can provide an additional sensitivity of up to 5% [118] to that of PCR alone. Colonization may occur, but a positive culture in combination with clinical suspicion should be considered pathogenic.
Serology has not been adequately evaluated in Mucorales infection. ID sensitivity has been reported at 73% [119] of culture-positive cases, with a specificity that approaches 100% [119]. Serology is not commercially available, however, and its role is uncertain as it is not known to provide additional sensitivity to culture alone.
The pooled sensitivity of PCR of either tissue, serum, or BALF specimens has been reported at 88% [118] of cases meeting either proven or probable EORTC criteria, and when PCR is combined with culture, the sensitivity can increase to 93% [118]. BALF PCR has been reported positive in 40% [20] and 100% [120] of culture-positive cases, but has also been reported to provide up to an additional 80% [120] sensitivity to culture. Serum PCR, however, does not add additional cases [120,121], and is not generally recommended. As Mucorales culture grows poorly, the addition of PCR may improve detection, thus both PCR and culture should be used concurrently to optimize the yield of diagnosis (Figure 2).
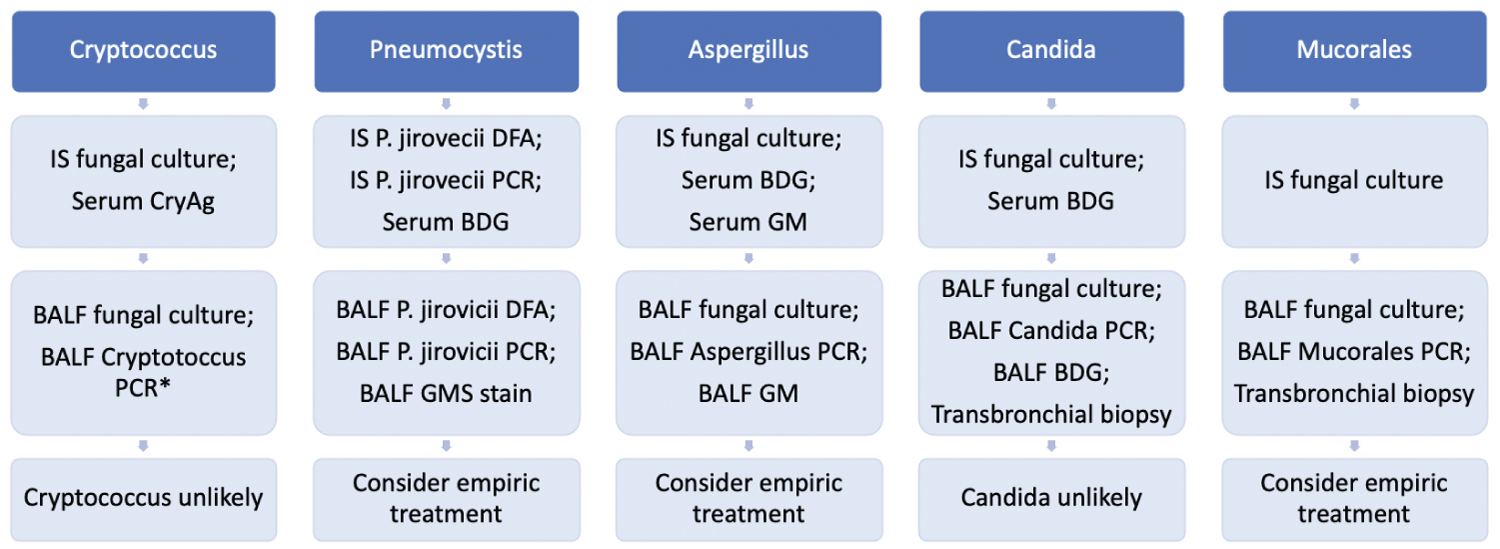 Figure 2: Opportunistic fungal pneumonia ("*" Indicates that the test is not widely commercially available).
View Figure 2
Figure 2: Opportunistic fungal pneumonia ("*" Indicates that the test is not widely commercially available).
View Figure 2
Changes in delivery of medical education as well as altered societal expectations have led to increased emphasis on laboratory testing over that of clinical expertise. Clinicians themselves increasingly rely on a laboratory or radiological finding more than they do their own clinical diagnosis. This approach may be acceptable so long as there is well-defined guidance and when there is acceptance and knowledge of the frequency of false-negative testing. Most endemic fungi such as Histoplasma, Blastomyces, and Coccidioides, as well as opportunistic Cryptococcus and Candida can be correctly identified with current laboratory techniques, however infection caused by Pneumocystis, Aspergillus, and Mucorales cannot be ruled out with current testing. Laboratory investigations should complement, rather than supersede, either clinical judgment or classic radiological findings.
All authors have contributed equally.
There is no financial support or funding to report.
There are no conflicts of interest to report by any author.