Coronavirus infectious disease 2019 (COVID-19) pandemic is a global public health issue due to a new coronavirus called severe acute respiratory syndrome virus 2 (SARS-Cov-2). It is a very contagious infectious disease with often benign symptoms. However, some patients present with severe clinical signs and are more at risk to die. The aim of this study was to assess the factors associated with severe COVID-19 in an Epidemic Treatment Center (ETC) at Dakar, Senegal.
We conducted a prospective descriptive analytical study dealing with COVID-19 patients treated in the ETC at the Military Principal Hospital of Dakar, from April 4, 2020 to November 2, 2020. Univariate and multivariate logistic regression were performed to identify the factors associated with severe COVID-19, which was defined as cases with oxygen saturation < 90% on room air.
During the study period, 238 patients with COVID-19 were enrolled. The mean age was 53 ± 18 years and male to female ratio was 1.38. There were six different nationalities, including 226 Senegalese (95%). Of those included, 17 (7.1%) were smokers and 126 (52.9%) had at least one comorbidity. These comorbidities included high blood pressure in 64 (26.9%), diabetes mellitus in 59 (24.8%), asthma in 16 (6.7%), heart disease in 8 (3.4%), obesity 5 (2.1%), chronic obstructive pulmonary disease in 2 (0.8%), and chronic kidney disease in 2 (0.8%). Main symptoms that patients presented were fatigue in 141 (59.2%), cough in 138 (58%), fever in 133 (55.9%), headaches in 123 (51.7%), myalgia in 110 (46.2%), dyspnea in 73 (30.7%), anosmia in 45 (18.9%), ageusia in 43 (18%), sore throat in 38 (16%) and nasal congestion in 25 (10.5%). The mean oxygen saturation on room air was 96% ± 6%. Patients with severe COVID-19 represented 35 (14.7%). Hydroxychloroquine and azithromycin were administered to 177 (74.4%) patients. The mean hospital days was 12 ± 5 days. We recorded 10 deaths, corresponding to a mortality rate of 4.2%. After a multivariate analysis, the factors independently associated with severe COVID-19 were: advanced age (OR = 1.05; CI 95% [1.01-1.08]), heart disease (OR=7.7; CI 95% [1.1-53.4]), the delay of diagnosis (OR=1.08; CI 95% [1.01-1.16]), and community origin of COVID-19 (OR=11.3; CI 95% [1.3-98.1]).
The identification of factors associated with severe forms of COVID-19 may help better manage high-risk patients and reduce their mortality.
Severe COVID-19, Associated factors, Dakar
In late December 2019, there was an outbreak of a new coronavirus, which emerged in the city of Wuhan, Hubei Providence in China, which was initially called by the World Health Organization (WHO) 2019-nCoV [1,2]. This virus, secondarily called severe acute respiratory syndrome virus 2 (SARS-CoV-2), is the cause of coronavirus infectious disease 2019 (COVID-19) [3]. COVID-19 was declared a public health emergency of international concern by the WHO on January 30, 2020 and called for aggressive actions from all countries [4]. On March 11, 2020, WHO declared COVID-19 a pandemic due to its worldwide expansion [5,6]. Africa experienced its first case of COVID-19 in Egypt, 7 weeks after the initial outbreak [7], and Nigeria was the first country in sub-Saharan Africa to report a case of COVID-19 on February 2020 [8]. It was on March 2, 2020 that the Senegalese health authorities confirmed their first case of COVID-19, which originated from France [8]. Since then, there has been a rapid increase in cases with a lull period between October and November 2020 and a surge since December 2020. On March 17, 2021, WHO declared 120,164,106 confirmed cases of COVID-19 since the beginning of the outbreak including 2,660,422 deaths. Africa had 2,958,224 confirmed cases, including 74,959 deaths. Senegal has had 36,996 confirmed cases, including 978 deaths [9]. COVID-19 patients present with mild/moderate symptoms in 55% and around 30% are asymptomatic [10]. Percentages of severe COVID-19 vary widely from one study to another, ranging from 10-25%, and critical forms are estimated at 5% [10-16]. Some factors associated with severe COVID-19, such as advanced age, male sex, and presence of comorbidities are described in the literature [12,13]. Few studies assessing factors associated with severe COVID-19 have been performed in Africa. We conducted this study to identify factors associated with severe COVID-19 in the Epidemic Treatment Center (ETC) at the Military Principal Hospital of Dakar, Senegal.
A prospective descriptive analytical study was conducted covering the period from April 4, 2020 to November 2, 2020. Our study population included all patients infected with COVID-19, who were hospitalized in the ETC at the Military Principal Hospital of Dakar. All patients with confirmed COVID-19 by specific real-time RT-PCR test of nasopharyngeal or oropharyngeal samples, who were at least 16 years of age were eligible to be enrolled in the study. Patients with suspicious symptoms of COVID-19 and those who had contact with a confirmed case of COVID-19 were tested. Patients unwilling to participate or with less than 24 hours of hospitalization were not included.
Data were collected from individual interviews with patients and from medical records. The sampling method was exhaustive taking into account all hospitalized COVID-19 patients. A tested questionnaire was created and trained investigators were in charge to collect socio-demographic information (including age, gender, nationality, address), evidence of travel, country origin, date of hospitalization, comorbidities, lifestyle (including smoking and marital status), delay of diagnosis (defined as the time interval between the onset of symptoms and the positivity of COVID-19 PCR test), clinical features, COVID-19 disease categories (community acquired cases, imported cases or positive contact cases), number of hospital days, treatment received, and outcomes (cured, died, or transferred).
Data were recorded in EPI INFO software (version 7.2.2.6), exported to Excel (version 15.13.3), and analysed using R software (version 4.0.3). According to the distribution, quantitative variables were represented using either means ± standard deviations (SD) or medians and their interquartile ranges (IQR). Qualitative variables were represented using frequency and percentages. After checking the distribution normality and the homogeneity of variances, the means were compared using either Student's t-test or Wilcoxon Mann Whitney test. The proportions were compared using Pearson chi-squared test, Pearson's Chi-squared test with Yates' continuity correction, or Fisher's exact test. Based on the latest WHO severity definitions (January 2021), we defined severe COVID-19 cases as patients with oxygen saturation < 90% on room air [17]. To identify the factors associated with severe COVID-19, we performed a univariate logistic regression then a multivariate logistic regression to calculate the adjusted odds ratios (aOR) and their respective 95% confidence intervals (95% CI). All independent variables with a p-value < 0.2 in the univariate model were introduced in the multivariate model, and a backward stepwise method was used to generate the final model. Hosmer Lemeshow's adequation test and interactions checking between independent variables were performed to validate the final model with p-value < 0.05. At the end of this procedure, independent variables were significantly associated with severe COVID-19 when the CI 95% of their adjusted OR excludes the number of 1.
Informed consent was obtained from all participants included in the study. Participants' confidentiality and anonymity were preserved, and adequate medical care and follow up were provided.
Of 243 COVID-19 patients hospitalized at the ETC at the Military Principal Hospital of Dakar, 238 (97.9%) were included according to the following flowchart (Figure 1).
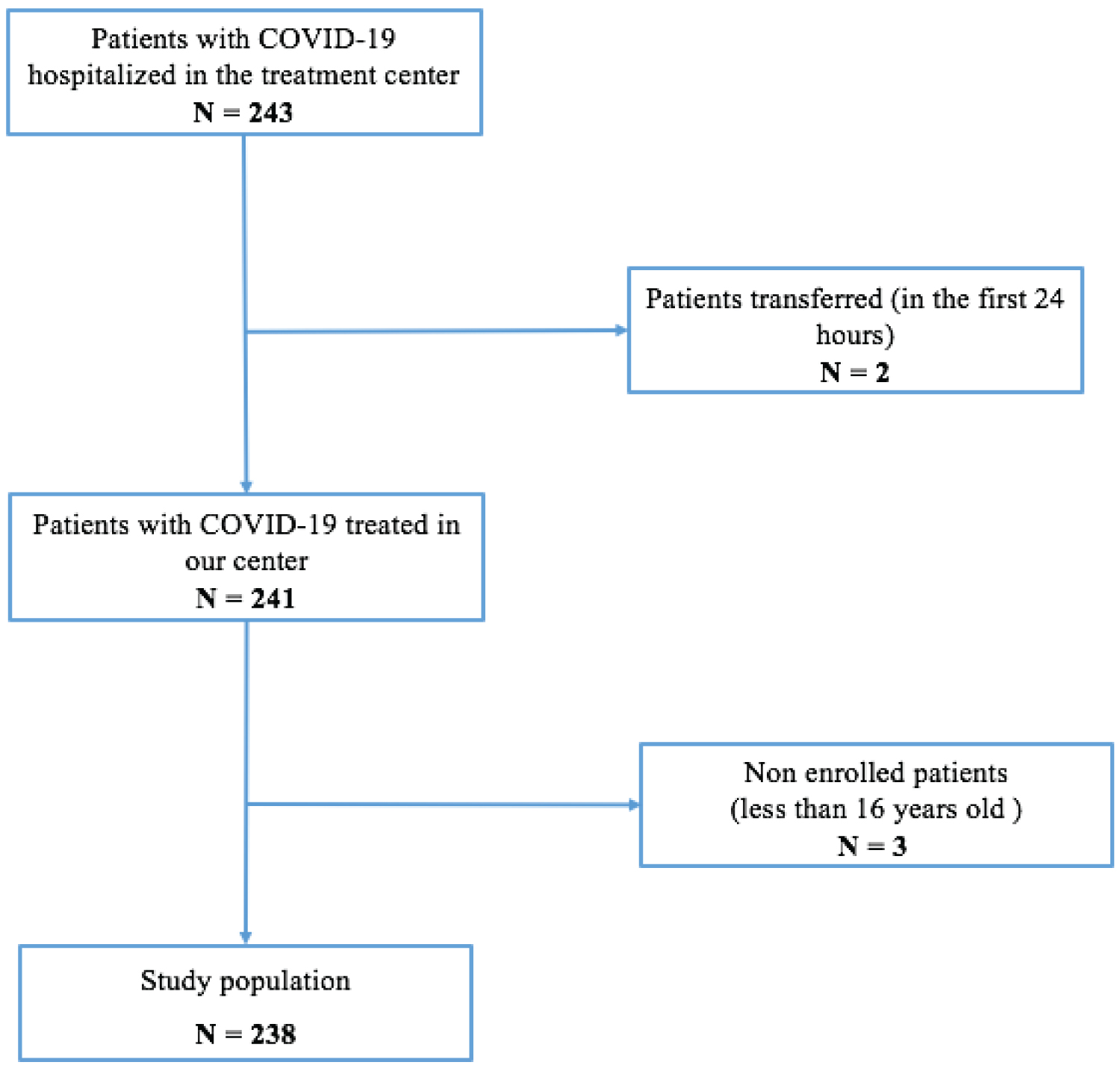 Figure 1: Flowchart of patients with COVID-19 in the ETC at the Military Principal Hospital of Dakar, Senegal, 2020 (N = 243).
View Figure 1
Figure 1: Flowchart of patients with COVID-19 in the ETC at the Military Principal Hospital of Dakar, Senegal, 2020 (N = 243).
View Figure 1
Of those enrolled, 165 (69.3%) were admitted through the Urgent Medical Aid Service, 60 (25.2%) were suspected cases, who were hospitalized and subsequently tested positive for COVID-19, and 13 (5.5%) were from COVID-19 Intensive Care Unit (ICU) after clinical improvement. The distribution of the patients by month of admission is represented in Figure 2.
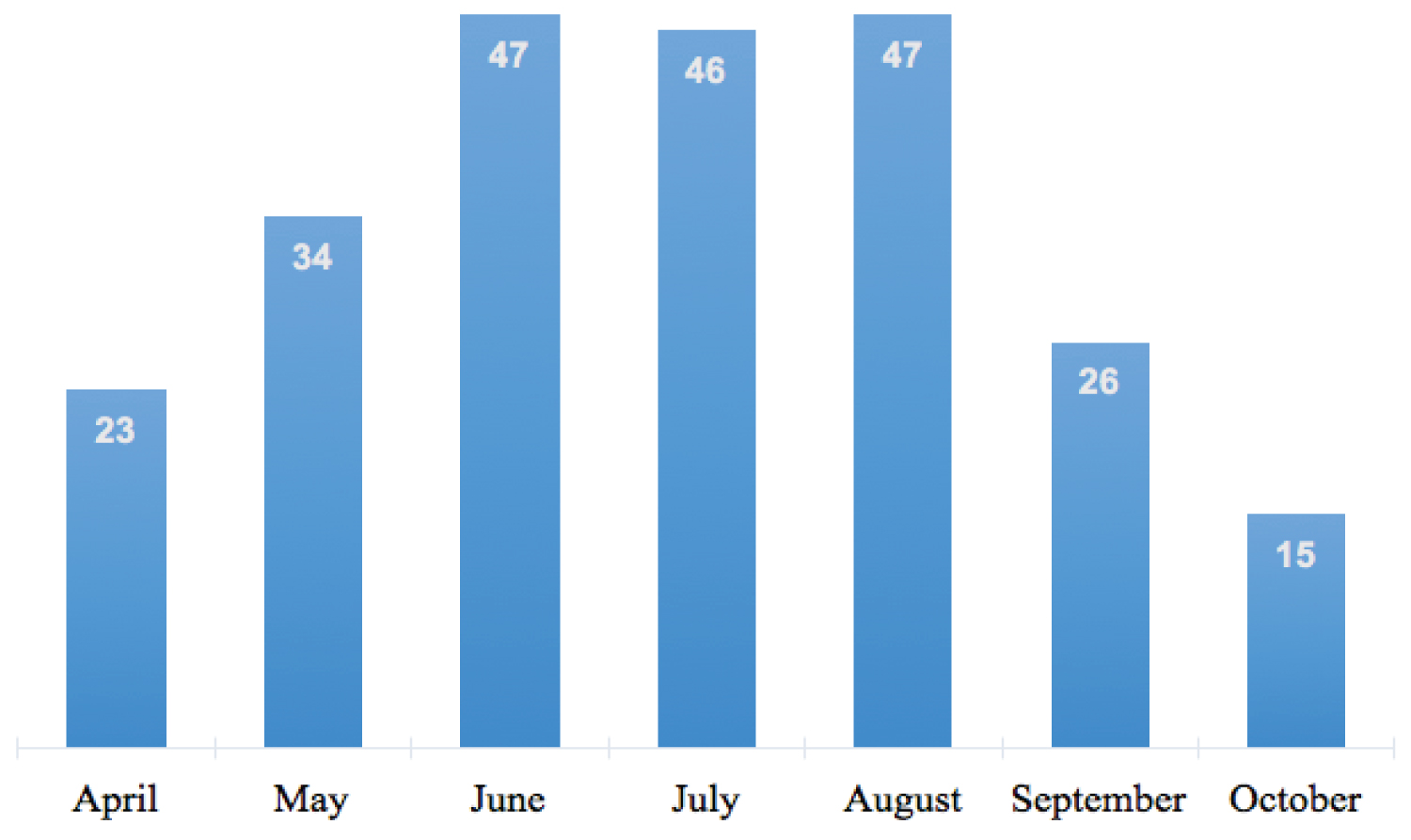 Figure 2: Distribution of patients with COVID-19 in the ETC at the Military Principal Hospital of Dakar, Senegal, 2020 (N = 238).
View Figure 2
Figure 2: Distribution of patients with COVID-19 in the ETC at the Military Principal Hospital of Dakar, Senegal, 2020 (N = 238).
View Figure 2
The epidemiological characteristics of the study population are represented in Table 1. The mean age was 53 ± 18 years and the male to female ratio was 1.38.
Table 1: Distribution of patients with COVID-19 according to their epidemiological characteristics in the ETC at the Military Principal Hospital of Dakar, Senegal, 2020 (N = 238). View Table 1
Of those included, 222 (93.3%) had COVID-19 symptoms. The main symptoms are represented on Figure 3.
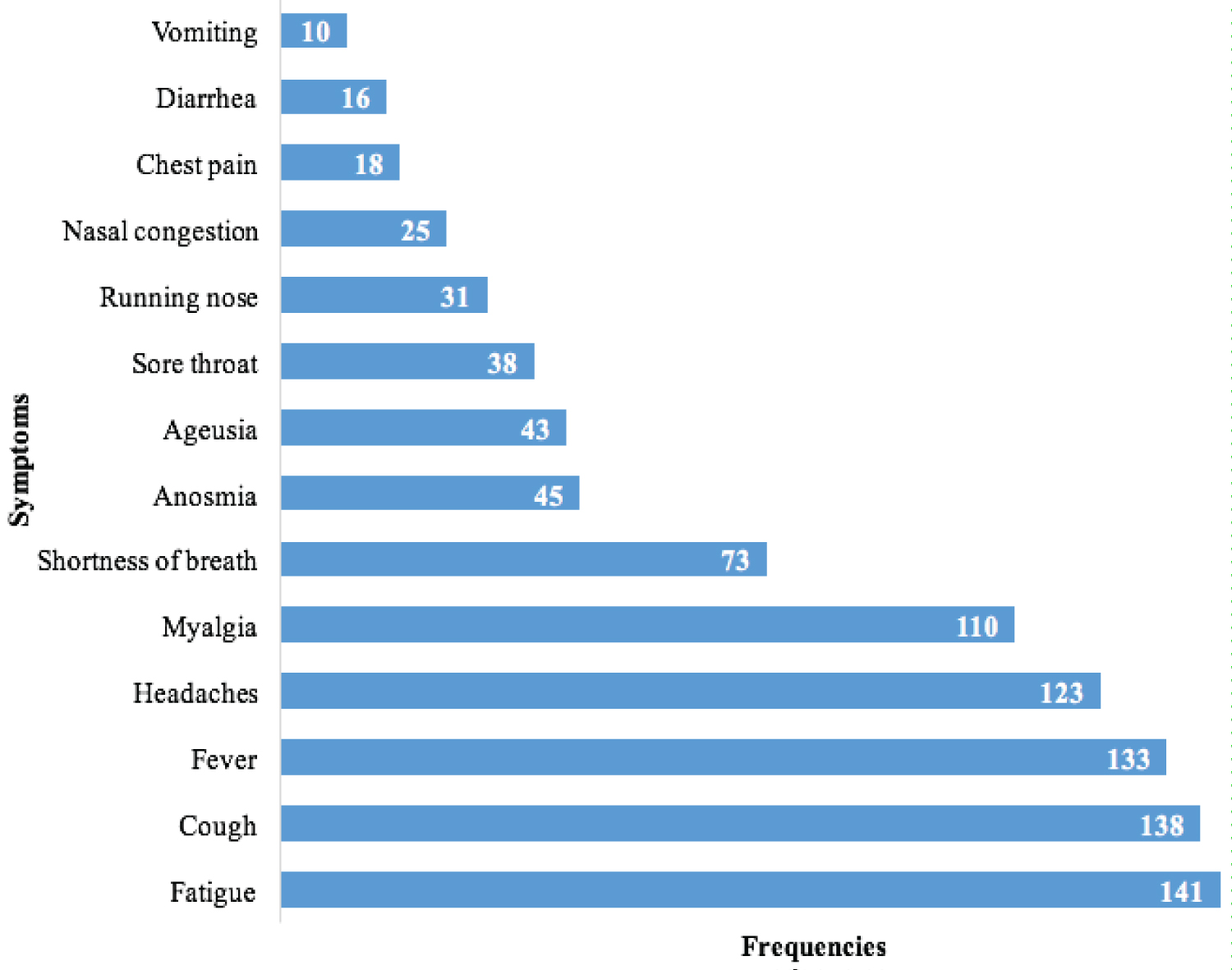 Figure 3: Distribution of patients with COVID-19 according to their symptoms in the ETC at the Military Principal Hospital of Dakar, Senegal, 2020 (N = 238).
View Figure 3
Figure 3: Distribution of patients with COVID-19 according to their symptoms in the ETC at the Military Principal Hospital of Dakar, Senegal, 2020 (N = 238).
View Figure 3
Severe COVID-19 cases were present in 35 patients (14.7%). The vital signs of the patients are represented in Table 2.
Table 2: Distribution of patients with COVID-19 according to their vital signs in the ETC at the Military Principal Hospital of Dakar, Senegal, 2020 (N = 238). View Table 2
The median delay of diagnosis was 6 days with (IQR = 4-9 days). There were 177 (74.4%) community-acquired cases, 60 (25.2%) cases with contacts who tested positive, and 1 (0.4%) case originated in another country.
In terms of treatment, 61 patients (25.6%) received symptomatic treatment while 177 (74.4%) received both symptomatic and etiological treatment (e.g. azithromycin + hydroxychloroquine). Among those who received etiological treatment, 21 (8.8%) presented side effects. The most common side effect was diarrhea in 13 (61.9%) patients.
The median hospital days was 12 days (IQR = 8-16). Fourteen patients (5.5%) were transferred to ICU during their hospitalization due to worsening clinical condition and 2 (0.8%) were transferred to other ETCs. Pulmonary embolism was present in 3 patients (1.3%). We recorded 10 deaths, corresponding to a mortality rate of 4.2%. Of 35 severe COVID-19 cases, 8 died, corresponding to a proportional mortality rate of 22.9%.
Epidemiological factors: In univariate analysis, the age groups of 45-65 years (p = 0.002) and over 65 years (p < 0.001) appeared to be significantly associated with severe COVID-19 compared to the group of age 16-45 years. The presence of at least one comorbidity was also associated with severe COVID-19 (p = 0.007). The different comorbidities that were significantly associated with severe COVID-19 were: Diabetes mellitus (p = 0.001), COPD (p = 0.02), and heart disease (p = 0.02). There was no significant association between the other epidemiological factors and the presence of severe COVID-19 (Table 3).
Table 3: Distribution of severe COVID-19 according to their epidemiological characteristics in the ETC at the Military Principal Hospital of Dakar, Senegal, 2020 (N = 238). View Table 3
Factors according to clinical classifications: In univariate analysis, a diagnostic delay of more than 7 days (p = 0.02) and community acquired COVID-19 (p = 0.01) compared to positive contact cases were significantly associated with the occurrence of severe COVID-19 (Table 4).
Table 4: Distribution of severe COVID-19 according to their clinical classification in the ETC at the Military Principal Hospital of Dakar, Senegal, 2020 (N = 238). View Table 4
After adjusting for potential confounding factors, the age groups of 45-65 years (aOR = 6.56; CI95% [1.3-33.1]) and over 65 years (aOR = 9.8; CI95% [1.9-51.4]) appeared to be significantly associated with severe COVID-19 compared to the group of age 16-45 years. The probability of presenting with severe COVID-19 was 7.7 times higher in patients with heart disease. This probability was multiplied by 1.09 (95% CI [1.03-1.15]) when the diagnostic delay was increased by one day. Patients with community acquired COVID-19 were 11.3 times more likely to present with severe COVID-19 (Table 5).
Table 5: Logistic regression assessing the associated factors with severe COVID-19 in the ETC at the Military Principal Hospital of Dakar, Senegal, 2020 (N = 238). View Table 5
During a period of 7 months, 238 patients with COVID-19 confirmed by RT-PCR, hospitalized in the ETC at the Military Principal Hospital of Dakar were enrolled in the study. Among them, 14.7% presented severe presentation of the disease. All potential explaining variables that were within our reach were taken into account in our analysis. After a multivariate logistic regression, associated factors of severe COVID-19 were: advanced age, heart disease, diagnostic delay and community acquired COVID19.
The prevalence of severe COVID-19 reported in our study (14.7%) is close to those found in Chinese studies: Wu Z, et al. (14%) [11] and Hui DS, et al. (15%) [13]. However, this is higher than that reported by Huang C, et al. (10%) in China [15] and lower than the result of Ouédraogo AR, et al. (23%) in Burkina Faso [12] and Mwana-Yile H, et al. (25%) in Congo [14]. The wide range of prevalence may be explained by the non-standardized definition of severe COVID-19 in different studies. It may also be related to the differences in the study populations, in terms of genetic, sociodemographic and clinical characteristics. Additionally, for a long time in Senegal, all patients who were tested positive for COVID-19 were admitted to the treatment centers regardless of their clinical presentation; this may have reduced the proportion of severe cases identified.
In our study, patients older than 65 years of age and those who were between 40 and 64 were 13.8 and 10.3 times more likely to present with severe COVID-19, respectively, compared to the patients aged 16 to 45. Similar results are described in all the studies available in the literature, evaluating the risk factors of severe COVID-19 [12,13,16,18,19]. This link between advanced age and the onset of severe COVID-19 may be due to the weakened immune system associated with advanced age, decreasing the strength to fight against the SRAS-CoV-2. This increased probability may also be due to other serious diseases related to age that may contribute to the occurrence of severe presentation of the disease.
Our study shows that patients with heart disease were 7.7 times more at risk to develop severe COVID-19. This is similar to the finding of Chen C, et al. in China [20], which showed that patients with known heart disease were 16 times more likely to have a severe COVID-19. Likewise, in a systematic review dealing with the relationship between cardiovascular disease and COVID-19, Yahia F, et al. showed that patients with both COVID-19 and heart disease were 5 to 10 times more at risk of severe forms and death from COVID-19 [21]. Two mechanisms may explain this association: COVID-19 can lead to acute pneumonia which may cause decompensation in those with pre-existing heart disease. SARS-CoV2 can also directly cause myocardial damage by binding to ACE-2 receptors, cardiac arrhythmias, acute coronary syndrome and venous thromboembolic disease [6,22]. Such cardiovascular events can lower oxygen saturation and lead to severe forms of COVID-19.
In our study, the probability of presenting with severe COVID-19 was multiplied by 1.09 (95% CI [1.03-1.15]) when diagnostic delay was increased by one day. Kaeuffer C, et al. showed in a prospective multicentre study carried out in France that the duration of symptoms was significantly associated with the occurrence of severe COVID-19 (p = 0.033) [19]. This relationship may be due to the fact that a delay in diagnosis and therefore in the management of COVID-19 may lead to the increased inflammatory injuries due to the COVID-19. Significant inflammatory injuries in the lungs can cause severe impairment of respiratory function.
The community origin of COVID-19, which we identified as a factor associated with the occurrence of severe COVID-19 compared to the positive contact cases (aOR = 11.3; CI95% [1.3,98.1]) has not been evaluated in studies available in the literature. Two arguments may explain this link. Firstly, patients with COVID-19 acquired from the community tend to be in denial of the disease and often do not rush to health facilities until the disease has advanced significantly. Secondly, the contact cases often do not present with severe symptoms at the time of diagnosis because they are tested early.
Some researchers have found other factors associated with severe COVID-19, such as high blood pressure [12], male gender [23], obesity, and diabetes [24]. All of these factors were taken into account in our analysis, but did not appear to be significantly associated with the occurrence of severe COVID-19. These disparities may be due to differences of study designs, sample sizes and study population.
Despite the results, which are globally in line with those found in the literature, our study has some limitations. The first is that the study enrolled participants from a single hospital center. This does not allow a statistical inference to the general population. The second is related to the relatively low sample size which reduces the strength of the study. The third is the definition of "severe COVID-19" which is different from that used in other studies. This may impact how our results compare to those of other authors. Finally, due to lack of laboratory investigations with which we faced at the beginning of the outbreak, we were unable to take into account in our analysis some important biological factors such as d-dimer, arterial blood gas measurement and troponin level.
This prospective study shows a similar prevalence of severe COVID-19 described in the literature. We have identified some factors associated with these severe clinical presentations such as advanced age, heart disease, delay of diagnosis, and community acquired COVID-19. A good assessment of the factors associated with severe COVID-19 through strong study designs may improve the management of high-risk patients and reduce their mortality.
The authors declare no conflict of interest regarding the publication of this paper.
We would like to acknowledge Jiyeon Jeon, MD, MPH (Providence Seaside Hospital, OR, USA) for the language and technical edits.
We acknowledge all the members of the ETC health- care team at the military principal hospital of Dakar for their involvement in the medical care of patients.