Hepatitis B infection (HBV) remains a significant clinical and public health problem and is hyperendemic in Nigeria. In highly endemic regions, infections spread from mother to child, or by horizontal transmission, with the burden of infection being highest in under-fives. Nigeria has a large number of orphans and vulnerable children, with reports of high seroprevalence of HBV infection in orphanages. There is no such report from our locality, despite having a high number of orphans. Therefore, this study was set to determine the risk factors, seroprevalence and infectivity of HBV in children resident in orphanages in Owerri, Southeast Nigeria.
A total number of 280 children below 18 years were proportionately recruited by multi-stage sampling. They comprised 140 children (73 females and 67 males) who lived in 10 orphanages and 140 sex and age-matched controls that lived in their family homes. Two milliliter (2 ml) of venous blood specimen was obtained from each child and the plasma tested for HBsAg and HBeAg using commercial quantitative and qualitatitive HBsAg ELISA kit and HBV-5 parameter rapid test kit respectively. Chi-squared test and odds ratio were used for data analysis; P-value < 0.05 was considered significant.
Seroprevalence of HBsAg in children in orphanages was 20.0% versus 10.7% in the controls (P = 0.031). Infectivity of HBV was higher in subjects (42.9%) than controls (20.0%) (P = 0.245). Sharing of towel (P = 0.014) sharing of barbing devices (P = 0.001) and circumcision (P = 0.005) were the significant risk factors for Hepatitis B virus infection in the orphanages; there was none in the controls. Male gender was significant for acquisition of HBV infection in the control group. Vaccination rate was lower in the subjects than the controls (P = 0.002).
There is high seroprevalence and infectivity of HBV in orphanages in Owerri, Southeast Nigeria. Sharing of towel and barbing devices and circumcision were the significant risk factors for HBV infection. There is need for continuous health education on Hepatitis B infection, improved standard of living and immunization coverage in orphanages in developing countries.
Hepatitis B virus, Risk factors, Seroprevalence, Infectivity, Children, Orphanage
Hepatitis B virus (HBV) infection is one of the most infectious diseases in the world and remains a significant clinical and public health problem due to its role in causing acute hepatitis, chronic hepatitis, a carrier state, hepatocellular carcinoma and death [1]. Hepatitis B virus, the prototype Hepadnaviridae, is a 42 nm partially double stranded DNA virus composed of a 27 nm nucleocapsid core surrounded by an outer lipoprotein coat containing three related envelope glycoproteins or surface antigens [2]. It is a hepatotropic virus that causes liver damage and dysfunction [3]. In acute infection, hepatitis B surface antigen (HBsAg) is the first serological marker to appear in the serum while the appearance of hepatitis B envelop antigen (HBeAg) in the serum usually indicates active HBV replication in the hepatocytes and infectivity [4].
Worldwide, about 257 million people are living with HBV infection, defined as hepatitis B surface antigen seropositivity [1], with 48 million children reported to be infected [5]. The prevalence of HBV infection varies between 2% (low) in developed countries and about 8% (endemic) in developing countries with Nigeria classified as hyperendemic [6]. In highly endemic countries, hepatitis B is commonly spread from mother to child at birth (perinatal transmission) or through horizontal transmission during the first 5 years of life [6]. Horizontal transmission occurs through close body contact with children, who have active infection or are in a carrier state, especially those with skin lesions like impetigo, scabies and cuts that enable the transfer of blood and body fluid. Horizontal transmission can also occur through sharing of toothbrushes, towels, beds, razors, needles, nail and hair clippers [1,7]. The risk factors for HBV infection include age, gender, geographic region, socioeconomic status, immunization status, lifestyle, sanitation and hygiene, and their relative contributions or influence to the transmission and prevalence of HBV infection are well documented in literature [8-10]. Certainly, HBV immunization is a cost-effective preventive strategy that has greatly reduced the prevalence of hepatitis in children [8].
In 2008, a study reported that 17.5 million children in Nigeria were orphans and vulnerable children (OVC) [11]. Imo State in Southeast Nigeria has a relatively high number of orphanages due to high number of OVC [12]. Most orphans and vulnerable children reside in orphanages (an orphanage is a residential institution devoted to the care of OVC) [13]. These children by virtue of their birth or environmental circumstances such as inadequate access to healthcare, poor sanitation and hygiene practices, overcrowding, poor level of care or supervision and increased risk of sexual abuse are at increased risk of HBV infection [14]. Children may acquire HBV through vertical transmission before admission into the orphanage, and within the orphanage may also have increased risk of horizontal transmission through exposure of cuts on skin (minor open wounds or mucosal surfaces) to blood or body fluids containing HBV. Older children who are sexually abused or sexually active may also acquire the HBV infection. HBsAg status of the child might not be known if medical screening is not routinely done prior to admission into orphanages. For those children who were abandoned, it is difficult to ascertain their hepatitis B vaccination status as well as that of their biological mothers. The hepatitis B status (HBsAg and HBeAg) of caregivers in orphanages may not be known before their employment and some of those who are carriers pose a risk to these children in orphanages mainly through infected bodily secretions.
Reports of seroprevalence of HBV infection in different parts of the world vary and studies [15,16] done in Nigerian children reported HBV seroprevalence of 0.5%-44.7%. There are limited data on infectivity (HBeAg) of hepatitis B in children in Nigeria in spite of the high seroprevalence of HBsAg reported. The seroprevalence of HBsAg and its infectivity (HBeAg) of children in orphanages and apparently healthy children in our locality is not known. This study was aimed at determining the seroprevalence of HBsAg, its infectivity (HBeAg) vaccination rate, and the risk factors that may predispose children to HBV infection in orphanages in Owerri, Southeast Nigeria. Early identification of these risk factors for transmission of HBV with appropriate management would reduce the burden of the disease. Knowledge of such findings might provide useful health related data on children in orphanages.
This study was conducted in Owerri, capital of Imo state, Southeast Nigeria. Owerri is made up of three Local Government Areas (LGAs), has an estimated population of 3.93 million and the inhabitants are predominantly Igbos, who are mainly civil servants, traders, farmers, artisans and students. Imo State had 22 registered orphanages as of the time of this study, 10 of them were located in Owerri. While some orphanages are managed by churches, others are supervised by individuals, Red Cross society and non-governmental organizations. The number of children in the various orphanages range from 10 to 45, though it fluctuates due to admission into the orphanage or adoption of the children.
This was a descriptive cross-sectional study carried out between June and August 2017. Inclusion criteria include: children younger than 18 years, who lived in all the 10 orphanages in Owerri. For comparison the control group comprised of children who were matched for age and sex and who lived in their family homes. Informed written consent was obtained from the care givers and parents/guardians. We excluded children for whom informed written consent could not be obtained.
Sample size was estimated using the formula for comparison of two proportions [17].
n =
Where n = sample size
p1 = prevalence of HBsAg in a previous study of 0.6% [18].
p2 = prevalence of HBsAg in a previous study of 7.6% [16].
cp.power = a constant defined by p value set at 0.05 and power of 80%
= 7.9
n
= 15.54 × 7.9
= 123
In order to accommodate possible errors with laboratory samples, attrition rate of 10% was built into the sample size. Thus 12 more subjects (10% of 123) were added to give 135, this was rounded up to 140.
One hundred and forty children residing in orphanages were recruited, while 140 children matched for age and sex were recruited as controls.
A multi-stage sampling method was used to recruit the subjects. All the ten orphanages in Owerri were selected for the study. The total number of eligible subjects was 192.
Stage 1: The number of subjects recruited from each orphanage was determined proportionately (Table 1) [19].
Table 1: Number of subjects proportionately selected from each orphanage. View Table 1
Example, if orphanage X has 35 subjects, number of subjects to be recruited from orphanage X will be
Where X is the number of children in orphanage X
T is the total number of children in all orphanages
Z is the calculated sample size
Stage 2: In each of the orphanage, simple random sampling (balloting) was used to select the subjects until the required number was met.
For the selection of the controls, households within a kilometer radius or households within the same ward/LGA (for orphanages located far from residential areas) where the orphanages were located, were identified and numbered. From each household, a control matching the subject in age and sex was randomly selected and where none met the criteria, or where parents/caregivers refused to give consent, the next household was selected. This process was done in each of the three local government areas. The number of controls selected from each household or LGA reflected the number of orphans (in the orphanages) found in that particular LGA.
A structured questionnaire designed for this study was used to obtain information from the study participants. Variables entered in the form include: socio-demographic data (patient's initials, research number, age, sex, address), HBsAg status of mother during pregnancy if known, the number of doses of vaccine received (verified through immunization records/cards for those available) and previous history of transfusion if any. Other risk factors such as scarification marks, circumcision, sharing of blades, sharing of toothbrushes, sponges and towels were obtained.
On physical examination, attention was paid to general physical appearance for the presence of palor, jaundice, scarification marks and on abdominal examination for presence of hepatomegaly and splenomegaly.
Two millilitre (2 ml) of venous blood was drawn from the children, ensuring standard precaution, into an appropriately labelled ethylene diamine tetra acetic acid (EDTA) bottle. Samples were temporarily stored and transported in a cold box to the laboratory. Blood samples were centrifuged at 4,500 rpm for 3 minutes to separate plasma from the red cells. The plasma was stored in a freezer at temperature of -20 ℃ until sufficient samples were pooled for analysis. Commercial quantitative and qualitative HBsAg ELISA kit (CTK Biotech) and HBV-5 parameter rapid test kit, for testing of HBeAg with same stored plasma was utilized, with strict compliance to the manufacturer's instructions in the manual and the standard operating procedure. Sensitivity and specificity of the ELISA kit were 100% and 100% respectively while sensitivity and specificity of the HBV-5 rapid test kit were 98.7% and 100% respectively.
CTK Biotech ELISA kit was used to test all samples for HBsAg, all positive samples were further tested with HBV-5 parameter rapid test kit for HBeAg. The plasma specimen was added to the antibody coated micro liter wells together with enzyme conjugated polyclonal antibodies. This was incubated at 37 ℃ for 60 minutes and then washed with phosphate buffered saline to remove unbound HBsAg. A solution of the substrate tetramethylbenzidine (TMB) was pipetted to the wells and incubated at 37 ℃ in dark for 15 minutes. Blue colour developed in proportion to the amount of HBsAg present in the specimen, while negative samples were colourless. The enzyme substrate reaction was stopped by adding ready-to-use-stop buffer (sulphuric acid) to each well and gently mixed for 30 seconds till all blue colour changed to yellow colour completely. The optical density (OD) of each well was determined within 15 minutes after adding the stop solution, using the spectrophotometer at 450 nm reading wavelength with 620 nm to 690 nm reference wavelength. The cut-off of plate was calculated using the manufacturer's instructions. The sample with the OD below the cut-off in each plate was considered non-reactive and therefore HBsAg negative while those with OD above or equal to the cut-off was considered reactive and therefore HBsAg positive.
HBsAg positive samples were tested for HBeAg with HBV-5 parameter rapid test kit. Two drops of the plasma specimen were pipetted into the circular groove of the cassette, followed by one drop of the buffer, results were read in 15 mins. If both C and T bands developed on the HBeAg groove, it was read as positive if C band was visible and T band was not visible the result was read as negative and if no red bands appeared or control line fails to appear, the result was interpreted as invalid and then retested.
A subject was considered infected with HBV (including past and current infection) if HBsAg positive, and not infected if HBsAg negative. A subject that is HBsAg positive and also HBeAg positive was considered highly infectious for hepatitis B.
The collected samples were temporarily stored and transported in an ice box to the laboratory for centrifugation after which they were stored in the freezer at a temperature of -20 ℃.
The ELISA kit and its reagents were stored in the refrigerator while the rapid test kits were stored in room temperature prior to use as directed by the manufacturers. The opened reagents that were not used immediately were refrigerated and brought to room temperature before use. Prior to analyses of the samples, ten samples were used to test run the incubator and ELISA microplate reader, adjustments were made to ensure correct results as indicated in the manufacturer's instruction. Strict compliance to manufacturer's instruction for the test kits was ensured.
Data was analyzed using the statistical package for social science SPSS version 20.0 for windows. The results were presented in tables. Continuous variable such as age was analyzed and expressed as mean, standard deviation, median and range while categorical variables were analyzed and expressed in proportions using frequency tables. Chi squared and Fishers Exact test were used as appropriate to compare and test associations between categorical variables. Odds ratio with 95% confidence intervals (95% CI) was used to compare HBV infection seroprevalence in both groups and to determine the relationship between HBsAg and infectivity (HBeAg). Multivariate logistic regression analysis was used to determine predictors of HBV infection, and P value < 0.05 was considered statistically significant.
A total of 280 children comprising 140 children residing in 10 orphanages in Owerri and 140 children (controls) living with their parents participated in the study.
Most of the study participants were below 60 months of age with 73 (52.1%) females and 67 (47.9%) males in each group, giving a male to female ratio of 1:1.1 (Figure 1A and Figure 1B).
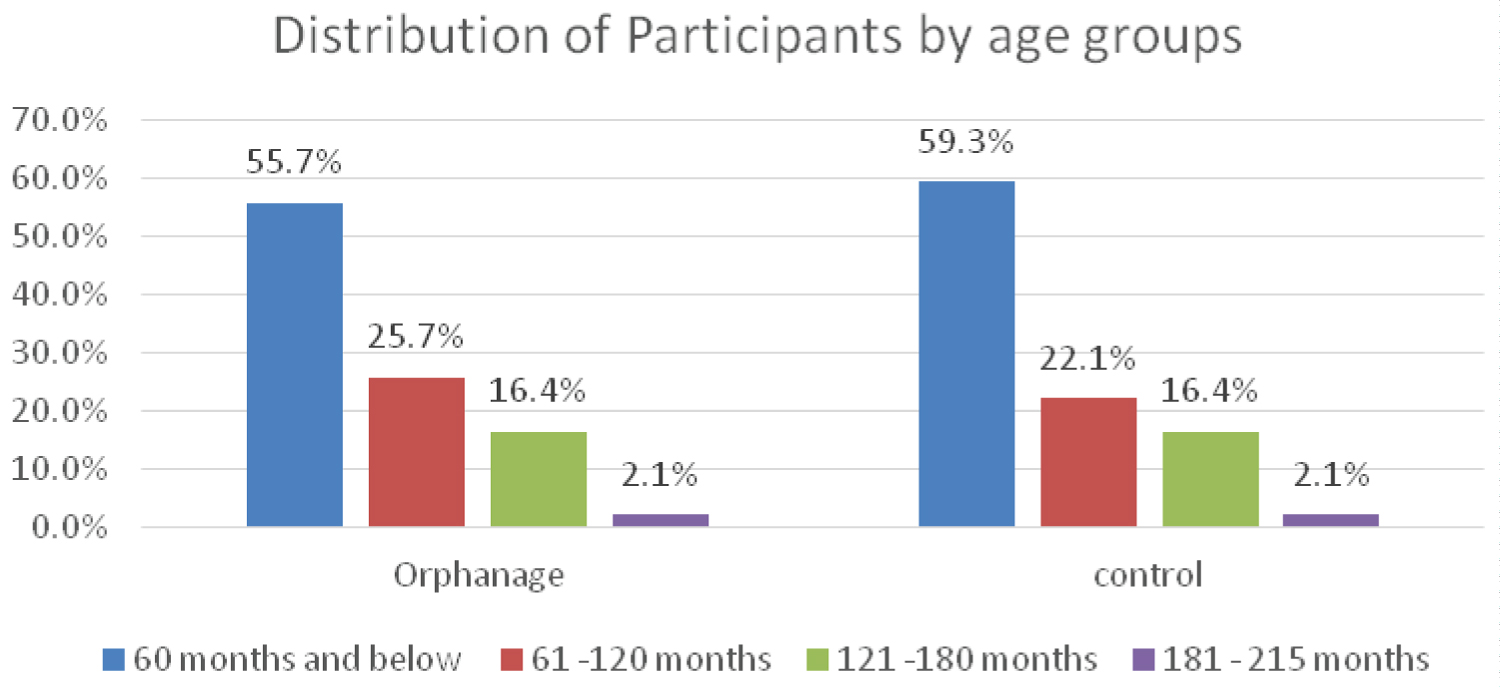 Figure 1A: Age group of study participants.
View Figure 1A
Figure 1A: Age group of study participants.
View Figure 1A
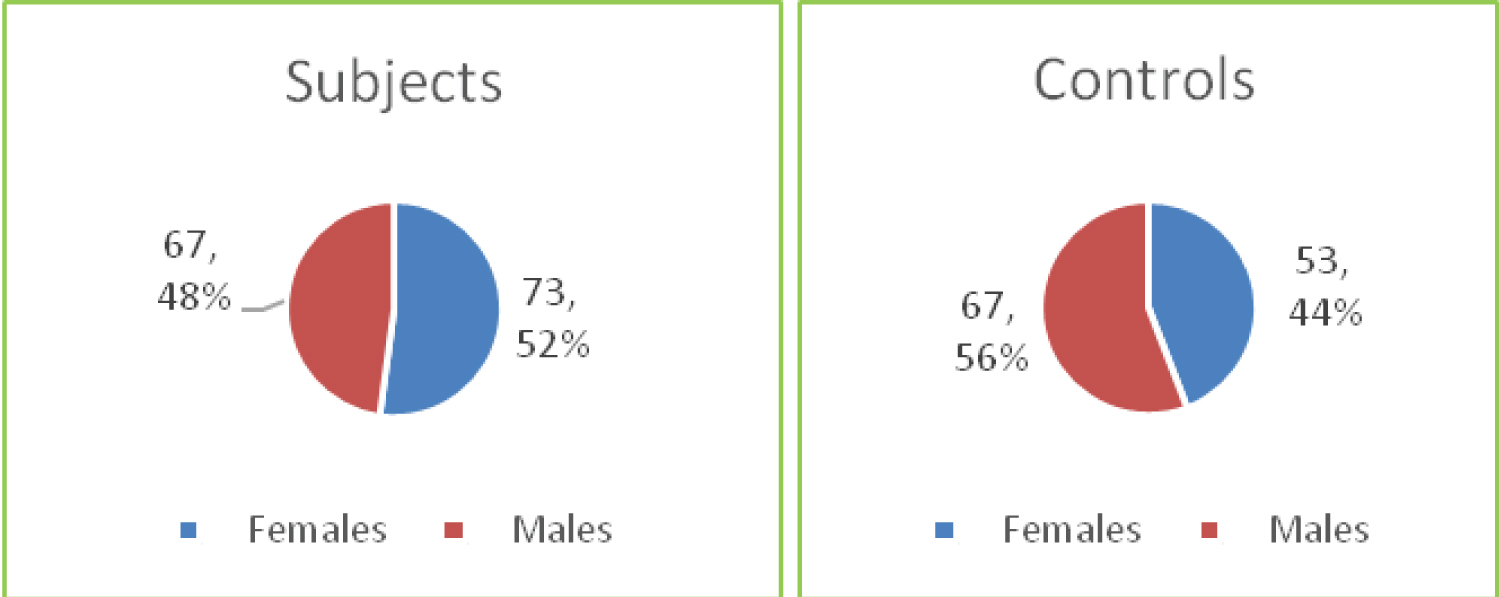 Figure 1B: Distribution of participants by gender.
View Figure 1B
Figure 1B: Distribution of participants by gender.
View Figure 1B
The seroprevalence of HBsAg was significantly higher in children in the orphanages compared to the controls (P = 0.031). The unadjusted odds of HBsAg seropositivity was twice as high in children in orphanage compared to the control (OR 2.080, 95% CI, 1.06-4.10) (Figure 2).
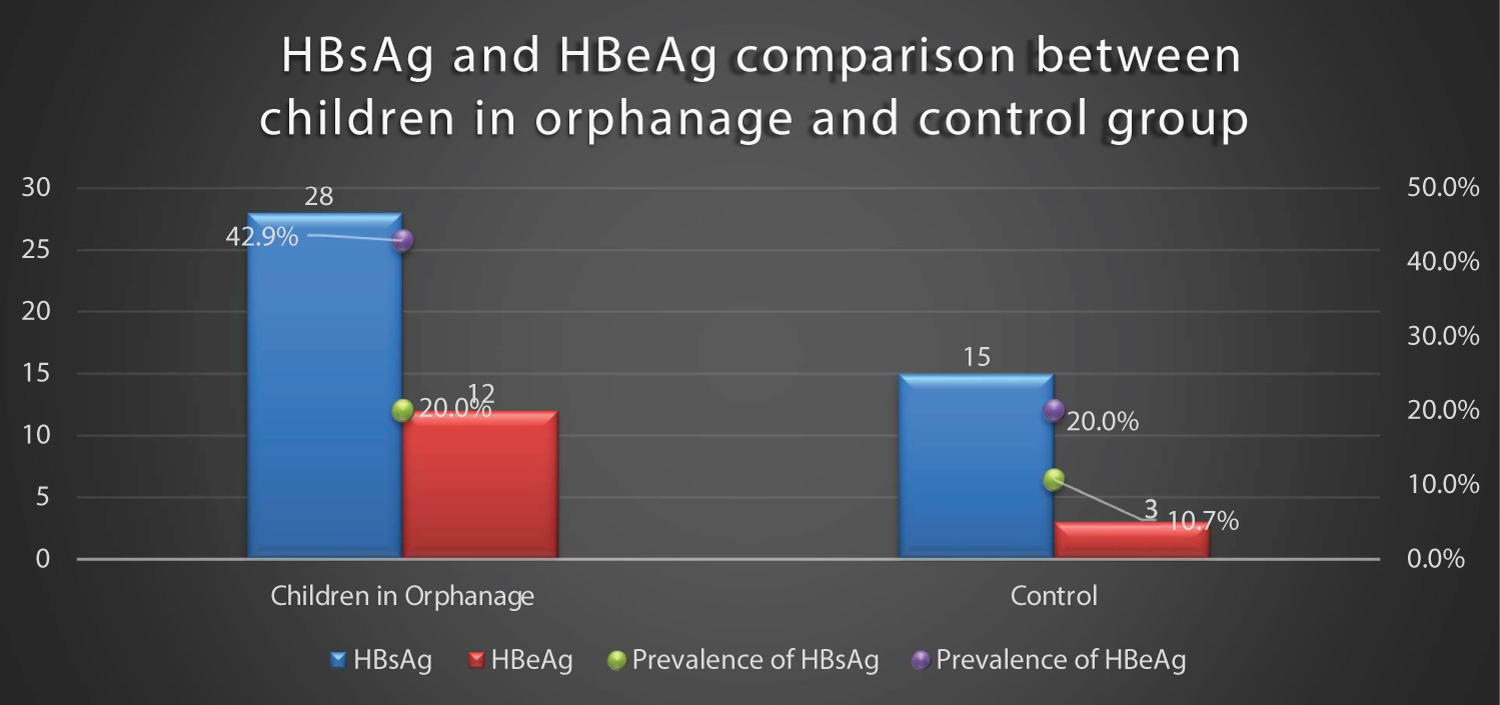 Figure 2: Prevalence of HBsAg and HBeAg of children living in orphanages and controls.
View Figure 2
Figure 2: Prevalence of HBsAg and HBeAg of children living in orphanages and controls.
View Figure 2
Amongst the children living in orphanages, the risk factors that were significant for acquisition of HBV include sharing of towel (P = 0.014), sharing of barbing devices (P = 0.001) and circumcision (P = 0.005) (Table 2).
Table 2: Association of HBV seropositivity with various risk factors in children living in orphanages. View Table 2
The significant risk factors for the acquisition of HBV infection were tested with multivariate logistic regression analysis; none was independently associated with HBV infection (Table 3).
Table 3: Multivariate logistic regression analysis for the predictors of HBV infection in the orphanage. View Table 3
There was no significant association between HBsAg seropositivity and various risk factors in children living with their parents (control group) (Table 4).
Table 4: Association of HBsAg seropositivity with risk factors in the controls. View Table 4
The difference in the vaccination status and number of vaccines received in both groups (subjects and controls) reached a statistically significant level, P = 0.002 and P = 0.001 respectively (Figure 3).
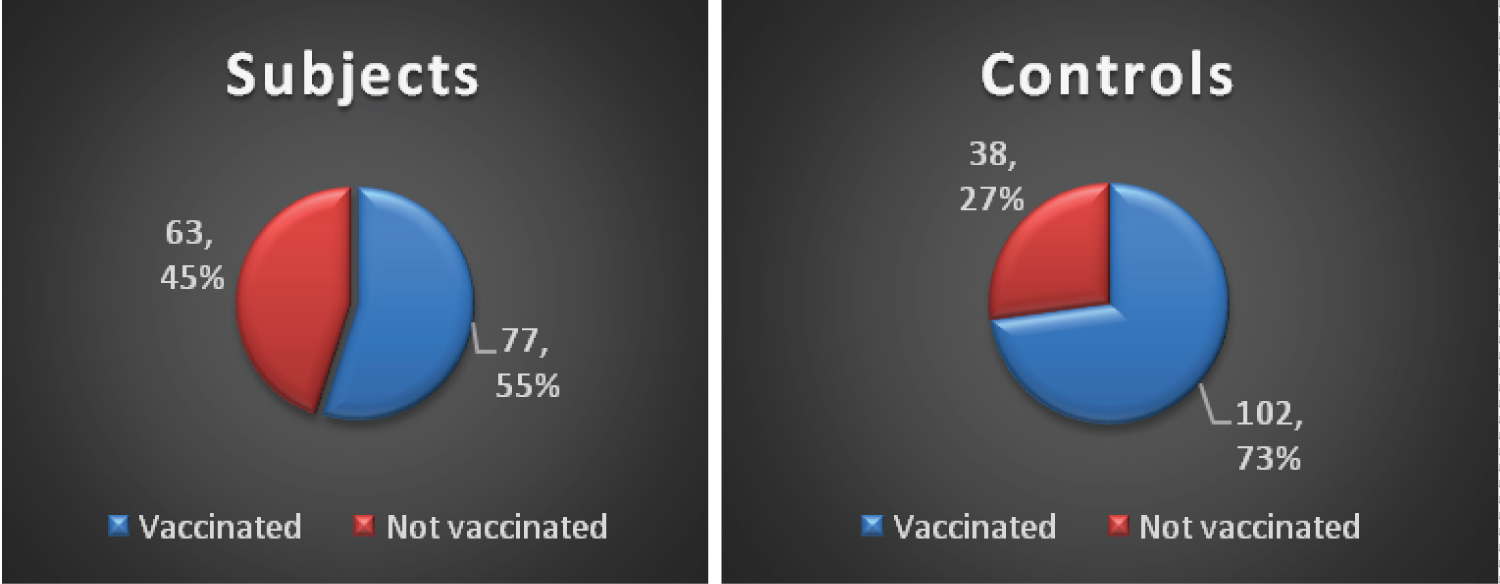 Figure 3: Comparison of HBV vaccination status of study participants.
View Figure 3
Figure 3: Comparison of HBV vaccination status of study participants.
View Figure 3
Absence of vaccination and incomplete vaccination were not significantly associated with HBsAg seropositivity in the study participants (Table 5).
Table 5: Relationship between vaccination Status and HBsAg status of the study participants. View Table 5
This study showed a significantly higher seropositivity of HBsAg in children resident in orphanages compared to children living in their family homes. The HBsAg seroprevalence of 20.0% found in this study is comparable to 20.8% reported by Haukenes, et al. [20] in an orphanage in Romania, but higher than 0.6% reported by Oluboyo, et al. [18] in orphanages in Anambra State. Time trend may explain the high prevalence recorded in Romanian study. It was done more than two decades ago when routine HBV immunization was yet to be implemented worldwide and the authors attributed the high prevalence of HBV infection found in their study to poor immunization coverage. More so, higher immunization coverage with first dose of hepatitis B in Anambra State of 75%, and 55% for Imo state as reported by National immunization coverage survey (South East zone) [21] may explain the lower prevalence of HBV recorded by Oluboyo, et al. [18] Poor immunization coverage, alone may not explain the high prevalence in this study. Inadequate access to healthcare, poor sanitation, overcrowding and poor general living conditions characterize most orphanages in developing countries and these could increase the risk of HBV transmission [14].
The HBsAg seroprevalence of 10.7% recorded among the control group is similar to 10.7% documented by Jibrin, et al. [22] in Sokoto, and Agbede, et al. [23] in Ilorin, who found a HBsAg seroprevalence of 10.0% in preschool children. The significant difference in HBsAg seroprevalence between the subjects and controls in this study could be as a result of absence of routine HBsAg screening of children admitted to orphanages and their caregivers during employment, and also the finding of lower rate of vaccination when compared with the controls. It was observed that most children were only screened for HIV and not HBsAg before admission and this might increase the chances of horizontal transmission from positive cases. The findings of this study are consistent with the results of earlier studies [20,24] and reinforce that HBV infection is relatively more prevalent in orphanages. Therefore, there is need for improved immunization coverage of orphanages, screening of babies and workers before admission and employment into orphanages respectively.
Seropositivity for HBeAg usually indicates active HBV replication in the hepatocytes and infectivity with implications for transmission, carrier state, chronic hepatitis B infection, hepatocellular carcinoma, cirrhosis and death [1,4]. This present study found a high HBV infectivity (HBeAg) of 42.9% in orphanages and 20.0% for controls. Though the difference in infectivity of HBV was not statistically significant, the odds of finding a child with HBeAg was three times higher in children in orphanages. The HBV infectivity obtained in this study is higher than 23.4% reported in Romanian orphanages. The higher seroprevalene of HBeAg in this present study could be attributed to practices observed within the orphanages such as sharing of sponge, towels, tooth brushes and sharp instruments which promote HBV transmission in developing countries.
Most of the burden of HBV infection is as a result of infection acquired before five years of age [1]. In this current study, children less than 60 months of age had the highest prevalence of HBsAg both in the subjects and controls Figure 4A and Figures 4B. Donbraye, et al. [25] in Osun State documented a similar finding. In contrast, Uleanya, et al. [26] in Enugu found higher seroprevalence of HBsAg in children above 5 years, which they attributed to effective Hepatitis B immunization and low risk of vertical transmission. The finding of under-fives having higher HBV seroprevalence than other age groups in this study may be attributed to vertical transmission before admission into the orphanages since high seroprevalence of HBsAg has been documented in pregnant women in Imo state [27]. Similarly in this study, HBeAg seropositivity was higher in the lower age groups in both study groups, a finding which is in keeping with that of Forbi, et al. [28] in North Central Nigeria and implies that these children have both higher chances of infecting others horizontally and chronic infection.
Females had a higher seroprevalence of HBsAg than males in the subjects though not significant, while more males than females had HBV infection in the controls, the difference reached a level of significance in the control group. Studies by Donbraye, et al. in Osun and Joanah, et al. in Calabar documented that females had a higher prevalence of HBsAg [25,29] while studies by Eke, et al. in Enugu and Bukbuk, et al. in Borno noted increased HbsAg seroprevalence in males [15,30]. The significant higher seroprevalence in males, might be attributed to their explorative lifestyles in terms of playing, and are thus prone to physical injuries, thereby increasing the risk of horizontal transmission from those positive for HBV [31]. It could also be as a result of circumcision in males which is a documented risk factor for acquisition of HBV [15,32] (especially when done by quacks with unsterilized instruments) and also a significant risk factor found in this present study and as such, could have accounted for the higher prevalence.
Several risk factors for HBV transmission have been identified in multiple studies [7-10,33]. This study has shown that the risk factors significantly associated with HBsAg seropositivity in children resident in orphanages include sharing of towel, sharing of barbing devices, and circumcision. Children who are seropositive with open cuts on their skin can spread the virus when sharing towels or barbing devices. Martison, et al. [7] in Ghana and Goh, et al. [34] in Singapore had earlier reported significant association between sharing of towel and acquisition of HBV while Nwokediuko, et al. [35] in Enugu State noted significant association between sharing of barbing devices and HBV infection. However, studies by Eke, et al. [15] in Enugu and Sallama, et al. [36] in Egypt found no significant association between sharing of towel/barbing devices and HBV infection. The sharing of toiletries and materials for personal hygiene/grooming depicts the low standard of living in orphanages and some homes. Therefore, the findings in this study reinforce existing knowledge and emphasize the need for improved standard of living in orphanages in developing countries.
Poor sterilization practices of the instruments for circumcision, or bleeding and ulceration from circumcision site might lead to increase in the transmission of HBV [15]. In this present study, circumcision was significantly associated with HBSAg seropositivity. This is similar to the reports by Eke, et al. [15] in Enugu and Ugwuja, et al. in Abakiliki [32]. Conversely, Angyo, et al. [37] and Sadoh, et al. [38] found no association with circumcision and HBV infection. It is likely that in this index study, these children were already infected with HBV prior to circumcision.
On multivariate logistic regression analysis, none of the risk factors found in this study was independently associated with HBV infection. Nevertheless, contributions of various significant risk factors obtained in this current study, agree with existing literature that horizontal transmission contributes significantly to acquisition of HBV in endemic regions [1,39].
HBV immunization is a cost-effective preventive strategy that has greatly reduced the prevalence of hepatitis in children [8]. In this index study, a significantly lower proportion of children in orphanages (55% vs. 73%) were vaccinated. Nevertheless a much higher percentage of those vaccinated 75.3% and 88.2% in orphanages and in controls respectively, were HBsAg negative. Ndako, et al. [40] in North central Nigeria, and Odusanya, et al. [8] in Western Nigeria, had earlier reported a higher prevalence of HBV in unvaccinated children. This underscores the fact that increased access to immunization is pivotal to decreasing the high prevalence of HBV. Despite a higher proportion of vaccinated versus unvaccinated children in this study, the seroprevalence of HBsAg remained high. Reasons for this finding are unclear. It is likely that birth doses of hepatitis B vaccine were not received timely, since most of the children were brought to the orphanage by different categories of people. It might also be attributed to possible mother-to-child transmission at delivery before the commencement of immunization or failure of the cold chain. However, there was no significant association between HBV vaccination status and HBsAg seropositivity in this study.
There is limited information on both the seroprevalence of HBsAg in children living in orphanages as well as the infectivity (HBeAg) of hepatitis B infection, in children in the general population.
There is high seroprevalence and infectivity of HBV in orphanages in Owerri, Southeast Nigeria. Sharing of towel and barbing devices and circumcision were the significant risk factors for acquisition of HBV in children resident in orphanages but none was independently associated with HBV infection. There is need for continuous health education on Hepatitis B infection, improved standard of living and immunization coverage in orphanages in developing countries.
The approval for the study was obtained both from the Ethics Committee of FMC Owerri and Ministry of Women Affairs, Imo State and it was conducted in compliance with the Helsinki declaration.
• Consent for publication: Not applicable.
• Availability of data and materials: Data generated or analysed during this study are included in this published article.
• Competing interests: The authors declare that they have no competing interests.
• Funding: None.
• Authors' contributions: CPO conceived the topic, and made substantial contribution to study design, data acquisition and drafting the original manuscript; CCO made substantial contribution to drafting the original manuscript and writing the final version; ANI, TCE, ECN and CBE were involved in the design of the study, revised the article critically for important intellectual content and gave the final approval of the version to be published; FCE was involved in data acquisition and analysis. All authors read and agreed to the final manuscript.
• Acknowledgements: We thank Dr. Benedict Nwogoh, John Okwara (PhD) all the matrons in charge of the orphanages and parents of the controls and study participants for their co-operation.