The association between war, conflict, massive force displacement and infectious diseases has long been established. The influx of large numbers of syrian refugees to Lebanon burdened the country's infrastructure on several levels, including the public health sector. The aim of this article is to evaluate the association between certain reportable infectious diseases, and the presence of Syrian refugees in Lebanon. Data, from non-governmental organizations and the Lebanese Ministry of Public Health- Epidemiological Surveillance Unit, National AIDS Program and National Tuberculosis Program was reviewed. Information about Syrian refugees, when available, was compared to Lebanese nationals. Several search engines were utilized to obtain literature relevant to infectious diseases associated with the presence of syrian refugees in Lebanon. For certain infections mainly tuberculosis treatment was provided to both the host and refugee communities. Whereas treatment for other infections such as viral hepatitis and brucellosis, was only available to the host community and not the refugees. Leishmaniasis outbreaks were exclusively linked to the presence of Syrian refugees and treatment was provided to the refugees to protect the host community. Outbreaks of vaccine preventable diseases were managed through intensification and better outreach of immunization campaigns for both communities. The measles, mumps, and viral hepatitis A outbreaks were partially attributed to the extra burden due to the presence of large numbers of refugees and their distribution in Lebanon.
Significant risks from transmissible infectious diseases to both the host community and refugees exist in connection with the Syrian crises. War, crisis, overcrowding, suboptimal sanitary infrastructure, and over stretched public health assets, when combined together, may lead to increase and spread of infectious diseases.
LMoPH-EUS: Lebanese Ministry of Public Health- Epidemiological Surveillance Unit; AIDS: Acquired Immunodeficiency Syndrome; UNCHR: United Nations High Commissioner for Refugees; NTP: National Tuberculosis Program; WHO: World Health Organization; HAV: Viral Hepatitis A; HBV: Viral Hepatitis B; HCV: Viral Hepatitis C; IgG: Immunoglobulin G; UNICEF: The United Nations International Children Fund; USAID: United States Agency for International Development; IOM: International Organization for Migration; MMR: Measles, Mumps, Rubella; USA: United States of America; CDC: Center for Disease Control and prevention; TB: Tuberculosis; MDR-TB: Multidrug resistance Tuberculosis
The total number of Syrian refugees in Lebanon, registered with the United Nations High Commissioner for Refugees (UNCHR), exceeded 1 million in 2016. It is believed that more than 40% of the Syrian refugees residing in Lebanon are non-registered leading to a total estimate of about 1.5 million [1]. This makes Lebanon the country with the highest number of refugees per capita in the world [2]. Syrians began fleeing to Lebanon as early as 2011, where the majority of these refugees, were not located in camps or tented settlements but rather spread all over the country [3]. This article will discuss the effect of the presence of these refugees on certain reportable infectious diseases in Lebanon.
A descriptive analysis was conducted to review the incidence of certain reportable infectious diseases between 2008 and 2019. Data rom the Lebanese Ministry of Public Health -Epidemiological Surveillance Unit (LMoPH-ESU), and National Tuberculosis Program (NTP) [3] was reviewed during the study period relevant to viral hepatitis A, B and C, measles, mumps, salmonellosis, brucellosis, tuberculosis, and leishmaniasis. Information about incidence over years among the population in general and Syrian refugees when available was obtained. A PubMed, EMBASE, Google Scholar, and COCRHANE database literature search was conducted using Lebanon, Syria, viral hepatitis A, B and C, measles, mumps, salmonella, brucella, tuberculosis, leishmaniasis, and refugees as key words. Results were discussed pertinent to each infectious disease entity and conclusions were drawn for the overall situation.
HAV is endemic in both Lebanon and Syria. A drop in HAV IgG sero-prevalence from 97.7% to 78% between 1982 and 2005 among Lebanese adults was documented, rendering them more susceptible to symptomatic clinical illness [4]. Since 2013, Lebanon experienced a major rise in the annual incidence of reported HAV infections from 5-18/100000 up to 34/100000 inhabitants [4]. Syrian refugees represented 14% to 18% of all reported cases between 2013 and 2018 [3].
The host community, as well as the refugees, suffer from deterioration in their livelyhood where, according to the International Labor Organization, around 170,000 Lebanese citizens has fallen below poverty line by 2015, while unemployment rate increased to 20%. This worsening in living conditions along with the absence of sanitary infrastructure in Syrian refugees concentration areas and poor sanitary infrastructure in the country might have facilitated the spread of the virus. The unusual dry weather and the drop in annual rain precipitation by more than half the yearly average can be another contributing factor [5]. As a result of this increase in HAV incidence, the Lebanese health authorities recommended the introduction HAV vaccination to the national immunization calendar. Lebanon has not integrated the vaccine against HAV earlier because only countries with epidemiological shift in HAV from endemic to intermediate endemicity were advised to do so [6]. Lebanon communicated the problem to the 67th World Health Assembly where a new resolution for HAV was set that includes plans to improve actions related to health promotion and prevention of viral hepatitis, encouraging and reinforcing immunization strategies [7]. The United Nations International Children Fund (UNICEF) and United States Agency for International Development (USAID) provided help for the country in improving and guarantying citizens access to inexpensive, safe and reliable water [8]. Figure 1 reveals trends in the incidence of HAV reported cases between 2008 and 2018.
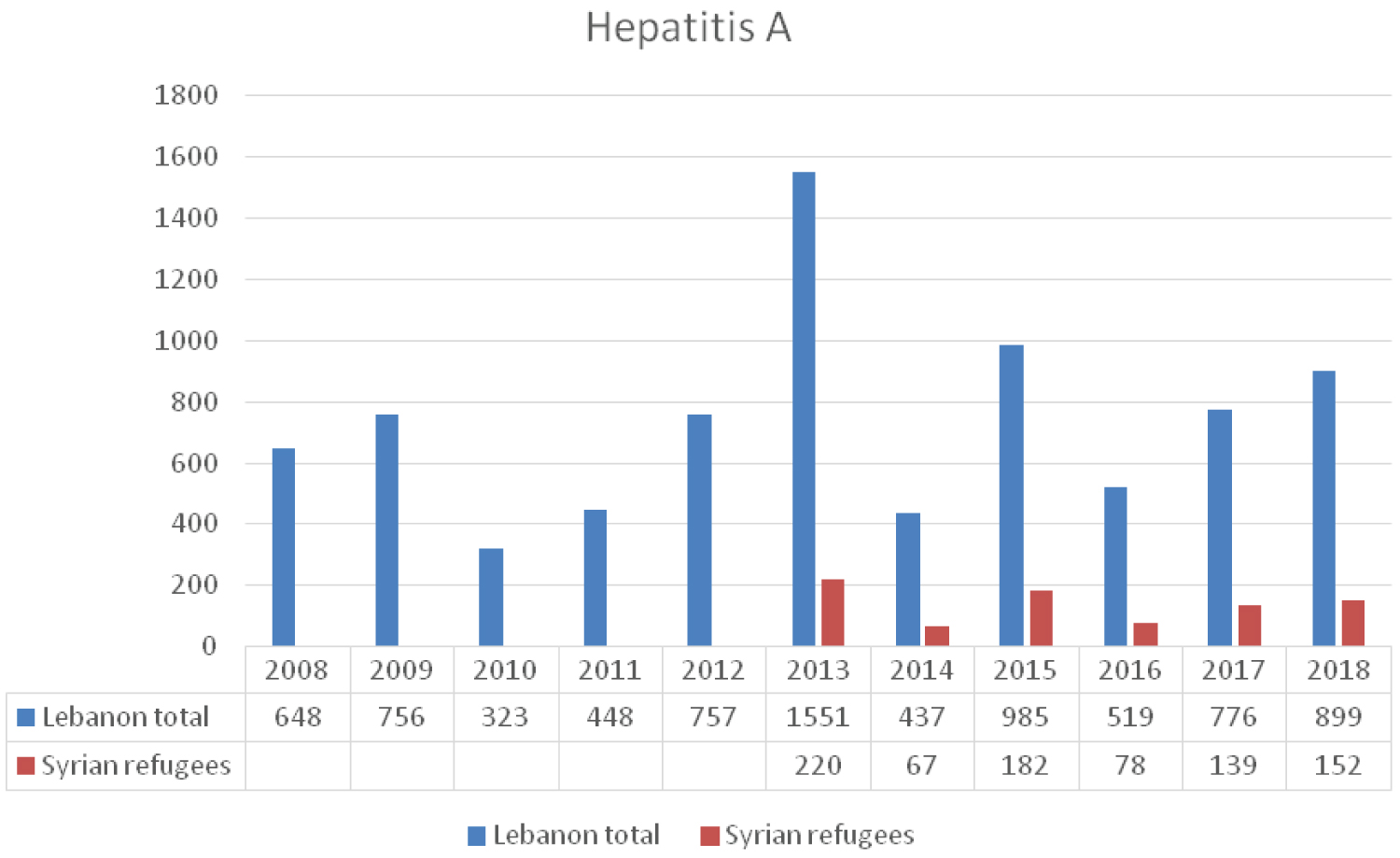 Figure 1: The reported HAV cases to LMoPH-ESU from 2008 till 2018 including Lebanese nationals and Syrian refugees.
View Figure 1
Figure 1: The reported HAV cases to LMoPH-ESU from 2008 till 2018 including Lebanese nationals and Syrian refugees.
View Figure 1
The prevalence of HBV in Lebanon is 1.74% [9] as compared to 5.6% in Syria before the war [10]. The average annual number of reported cases to the LMPH-ESU between 2008 and 2018 is 250 (Figure 2). An average number of 38 cases per year was reported among Syrian refugees residing in the country between 2013 and 2018. According to studies from blood banks carried out by the Syrian Ministry of Health, HBV seroprevalence was 2.66% and the vaccination rate was 83% in 2008. In 2011, with the start of the Syrian war, seroprevalence was 1.75% and HBV vaccination coverage rates dropped to 69% [10,11]. In a study at GeoSentinel clinics conducted in eight countries between June 2011 and November 2015, the status of hepatitis B and C was examined in 44 adult Syrian refugees, HBV infection was documented in 6.8% indicating that screening protocols for adults should address this infection and allocate the resources need for screening, treatment and follow-up [12]. A retrospective review of data was conducted between April 2014 and December 2015 in Turkey on 171 Syrian children aged between 0-18 years who were admitted for reasons other than jaundice, to the outpatient infectious diseases clinics. Six of the 140 patients (4.2%) were HBsAg positive, and all of these patients were anti-HBc total positive and anti-HBs negative [13].
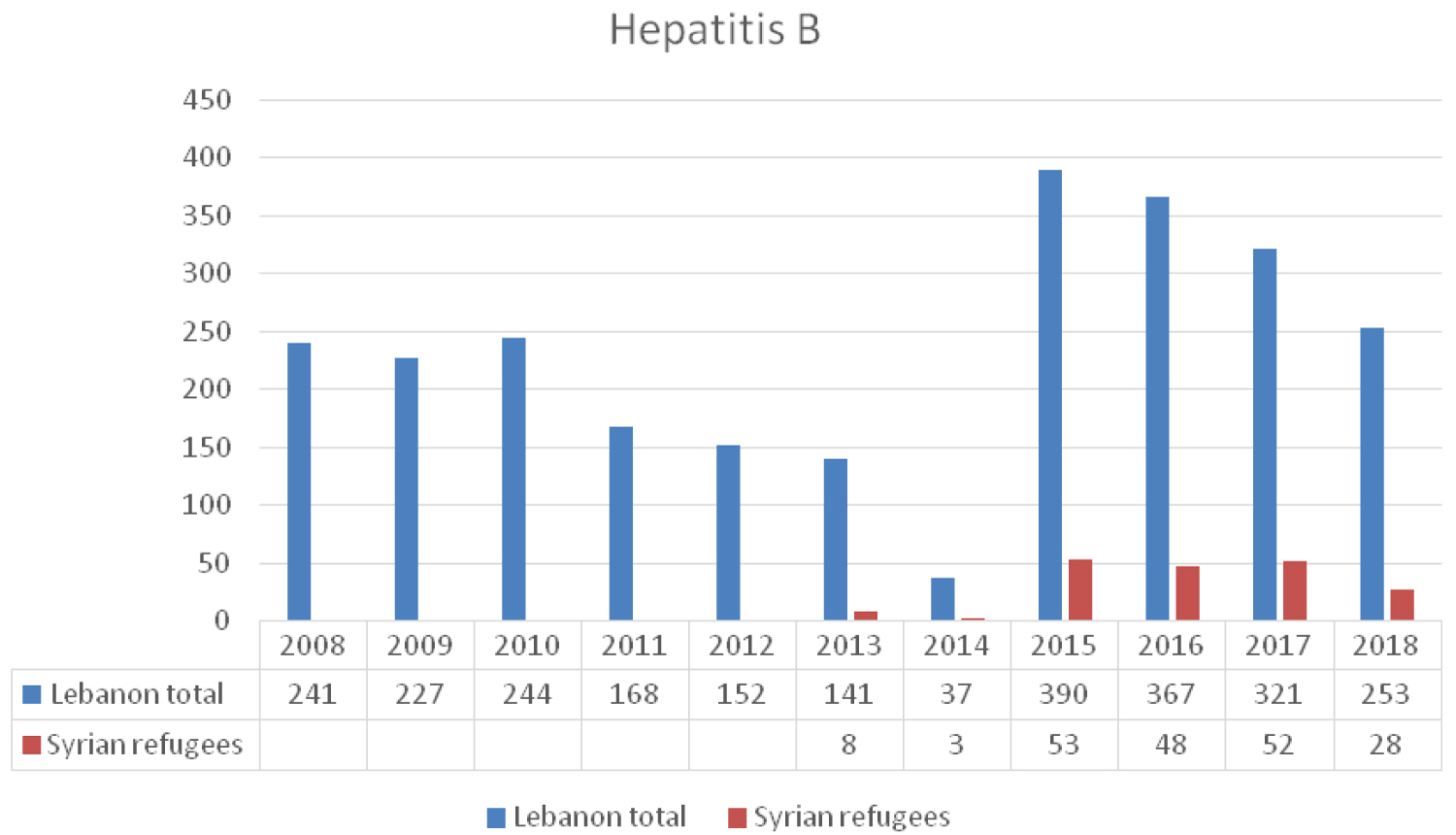 Figure 2: The reported cases of HBV infection to LMoPH-ESU from 2008 till 2018 including Lebanese nationals and Syrian refugees.
View Figure 2
Figure 2: The reported cases of HBV infection to LMoPH-ESU from 2008 till 2018 including Lebanese nationals and Syrian refugees.
View Figure 2
Anti-viral therapy for chronic HBV infection, when indicated, is provided free of charge to all Lebanese individuals who qualify for treatment. Non-lebanese including Syrian refugees residing in the country are not linked to any similar program of care. This may allow the virus to circulate undetected among this group, making it difficult to assess its impact and potential complications. The LMoPH provides free immunization coverage, including HBV vaccines, for children of Syrian refugees similar to that of the Lebanese children [14].
Two main genotypes of HCV predominate in the Middle East region G4 and G1 [15]. The prevalence of HCV in the general population is estimated to be around 0.2% in Lebanon and 0.4% in Syria. Among the lebanese nationals the distribution of genotypes suggests that G1 is the most prevalent (39.9%), followed by G4 (32.1%). As for the Syrian population G4 is most common (58.2%) followed by G1 (29.5%) [16]. In Turkey, the seroprevalence of HCV among Syrian refugee children was 1.8% as compared 0.1% in Turkish children [13]. The seroprevalence of hepatitis C at the GeoSentinel clinics was 2% which accords with the turkish data and the expected range already known in Syria [12].
The new directly acting anti-retroviral agents are dispensed free of charge only to Lebanese individuals infected with the virus. Syrian refugees are not included in the LMoPH plan of free access to treatment. Data from the LMoPH-ESU [3] about the distribution of reported cases of HCV infection (Figure 3) reveals only few cases among Syrian refugees. This low incidence might be due to the fact that they are not linked to medical care following diagnosis and as such not reported the LMoPH-ESU. This may allow the virus to circulate freely among refugees leading to fatal complications including advanced liver disease, hepatocellular carcinoma and cirrhosis [17].
The annual number of reported cases of measles increased from only 9 cases in 2012 to 1760 in 2013 with 82% being Lebanese and 13% Syrians [18] (Figure 4). Those less than 10 years of age were mostly affected representing 79 % of all cases. Most of the patients were either not vaccinated or their vaccination status was not known [18]. The efficient transmissibility of measles virus along with overpopulation and unsanitary conditions make the Syrian refugees highly susceptible to this infection [19]. Several vaccination campaigns were conducted to immunize both Lebanese and Syrian children. In 2014, as part of the fourth national integrated immunization campaign, 1,165,871 children were vaccinated against measles and a steep decrease in the number of cases was evident [18,19]. In 2014, UNICEF, in support of LMoPH, held a nationwide vaccination campaign and focused on routine immunization of lebanese and Syrian children. The number of reported measles cases dropped by the end of year 2016 to 44. However, the number of measles cases resurged again in 2018 to reach 938 cases (Figure 4). The majority were either unvaccinated or didn't receive the second dose of the vaccine [20]. Measles reemergence is a good example of the need to revisit immunization national policies in view of the emerging migration. Outbreaks of measles have been documented in migrant populations in Europe [21] and the International Organization for Migration (IOM) has recommended vaccination for recently arrived migrants/refugees [22]. A recent study showed a striking variation in policies on optimum approaches to vaccination in migrants across Europe. There was a call for more research and data collection, and need for dissemination of migrant-specific guidance [23].
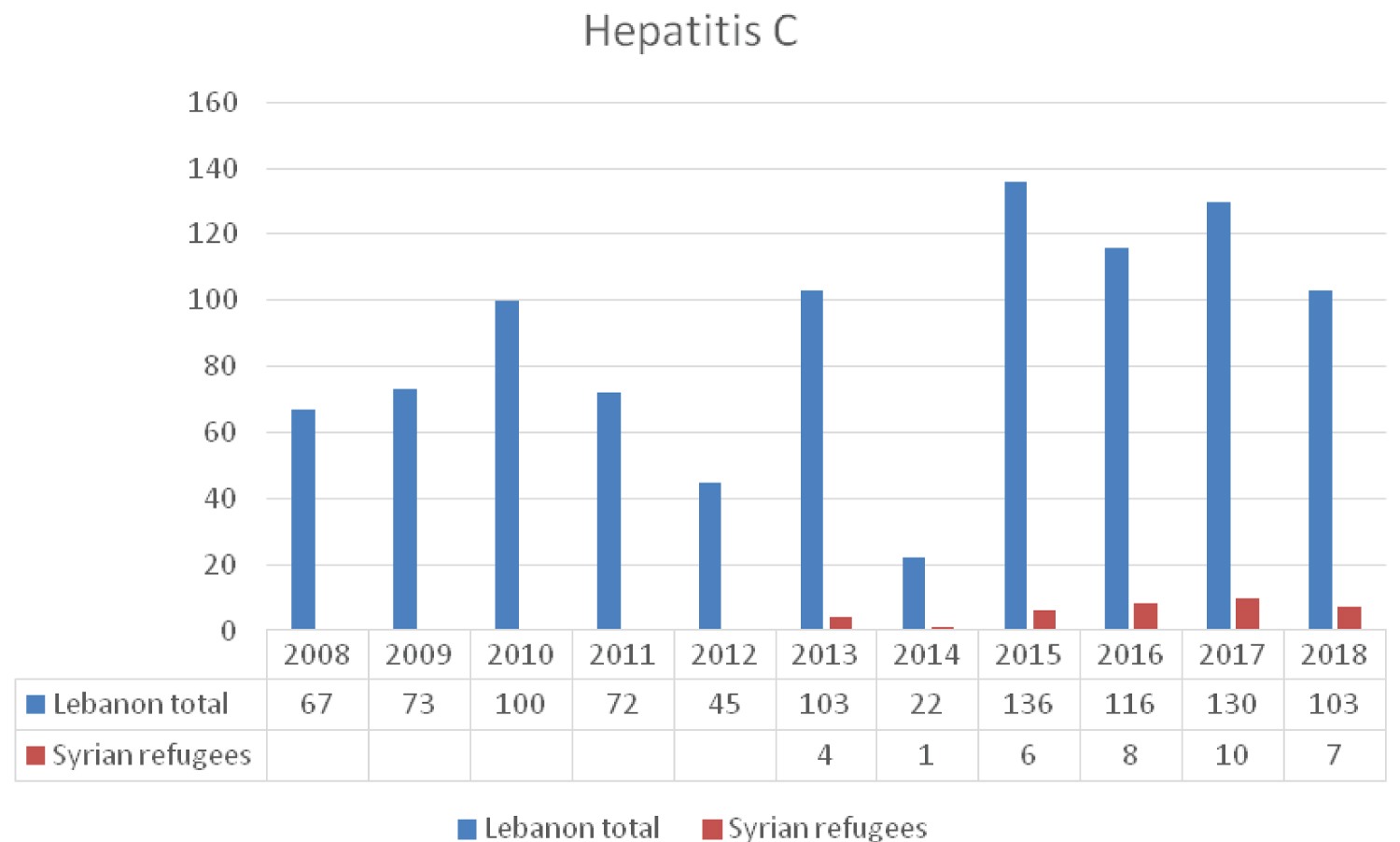 Figure 3: The reported cases of HCV to LMoPH-ESU from 2008 till 2018 including Lebanese nationals and Syrian refugees.
View Figure 3
Figure 3: The reported cases of HCV to LMoPH-ESU from 2008 till 2018 including Lebanese nationals and Syrian refugees.
View Figure 3
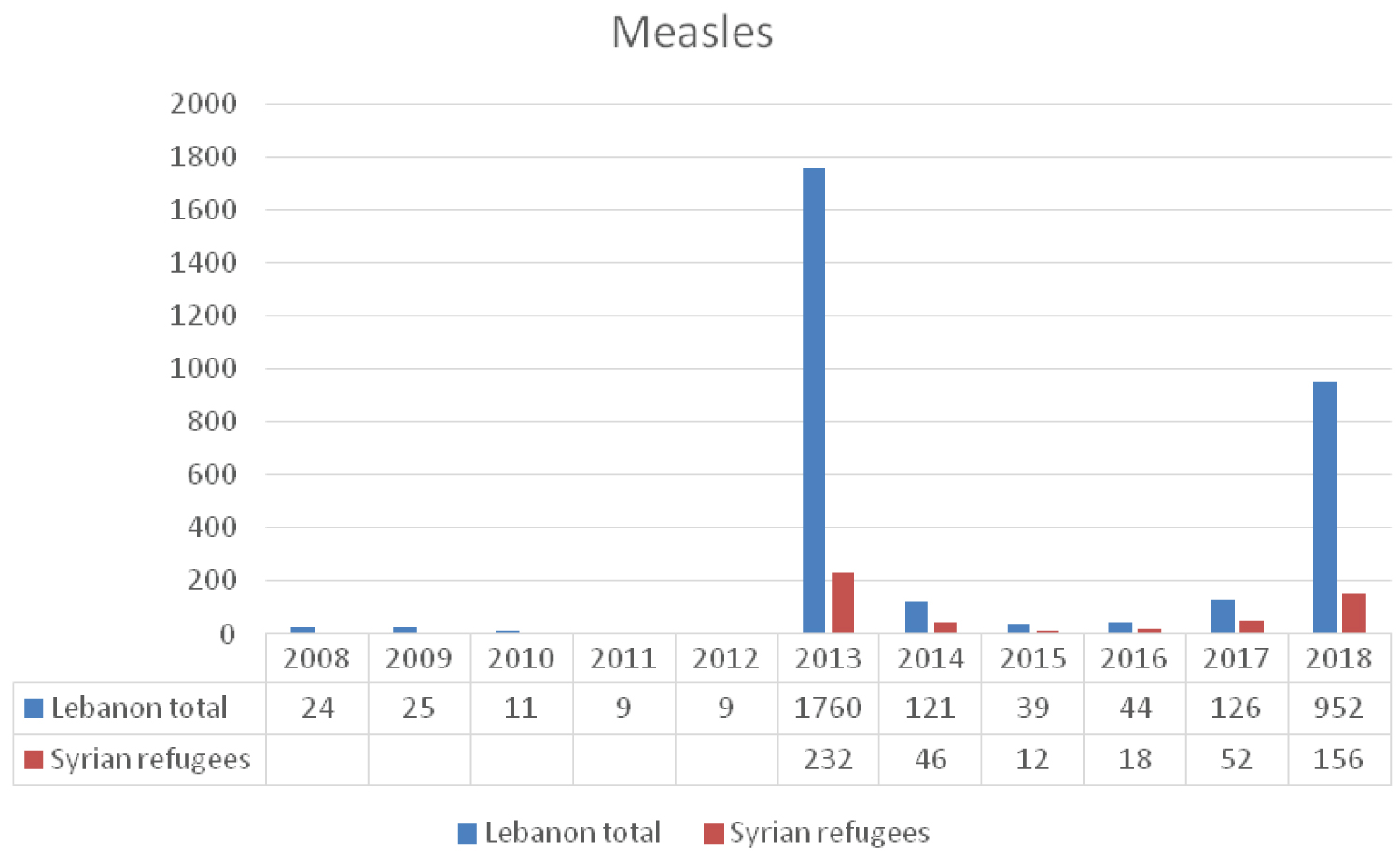 Figure 4: The reported cases of Measles to LMoPH-ESU from 2008 till 2018 including Lebanese nationals and Syrian refugees.
View Figure 4
Figure 4: The reported cases of Measles to LMoPH-ESU from 2008 till 2018 including Lebanese nationals and Syrian refugees.
View Figure 4
In 2014 and 2015 there was a nation-wide outbreak of mumps when 1320 cases were reported to the LMoPH-ESU (Figure 5). The majority of cases occurred in BeKaa, district close to the Syrian borders, where large number of Syrian refugees reside. This mumps outbreak occurred two years after the measles outbreak of 2013 [18]. The LMoPH immunization schedule against the MMR was modified where the first dose is introduced at 12 months of age and the second at 18 months. This variation from the USA schedule was mitigated by the recent measles outbreak of 2013 [14].
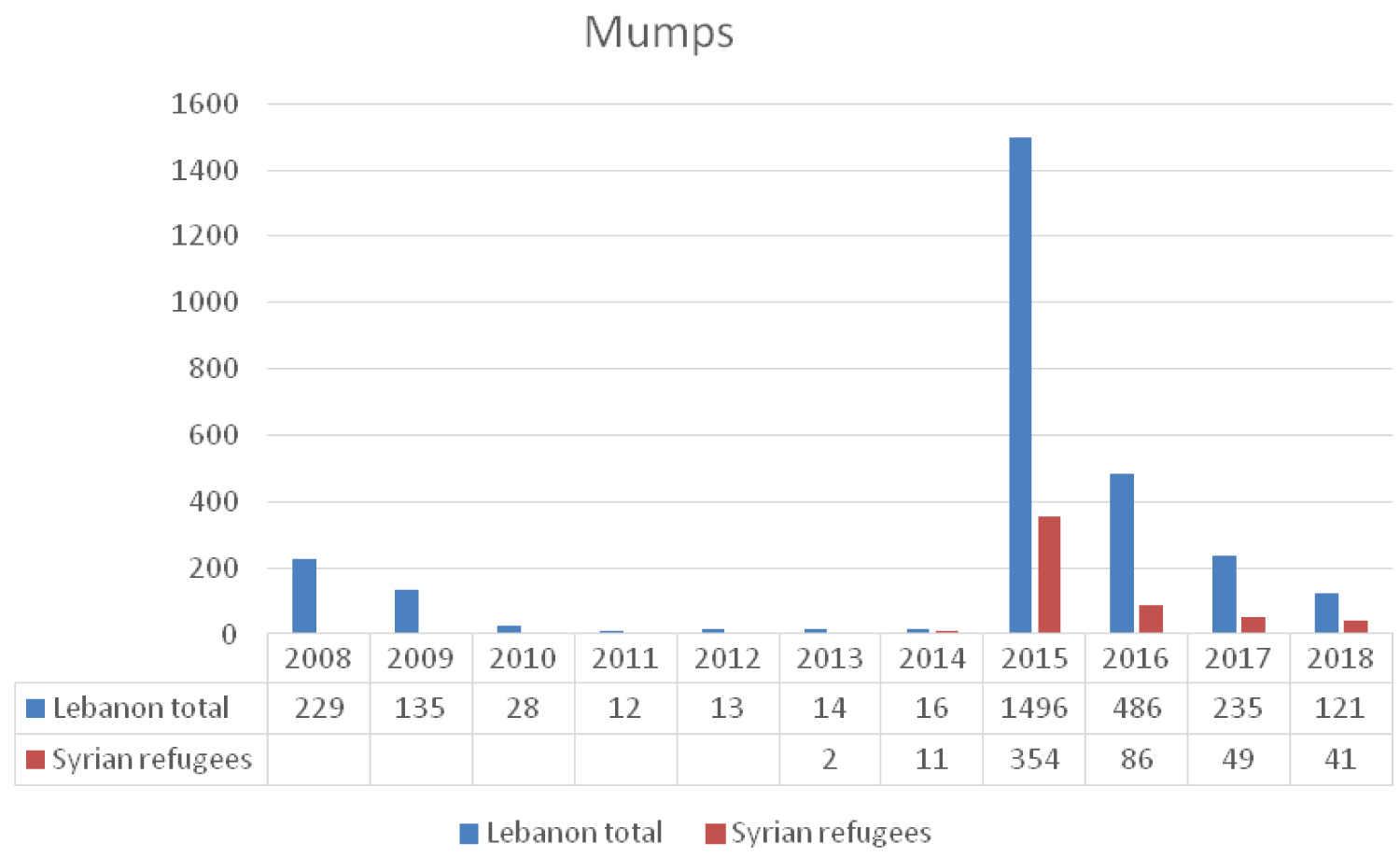 Figure 5: The reported cases of Measles to LMoPH-ESU from 2008 till 2018 including Lebanese nationals and Syrian refugees.
View Figure 5
Figure 5: The reported cases of Measles to LMoPH-ESU from 2008 till 2018 including Lebanese nationals and Syrian refugees.
View Figure 5
Despite the high number of Syrians in Lebanon, the number of reported mumps cases was relatively low [18]. This may be explained by either the good coverage immunization rates in Syria before the crisis or due to the lack of proper surveillance efforts by the Lebanese authorities (Figure 5) [18]. In 2015, a surge in mumps cases was noticed reaching 1496. However, as of 2016, the number of reported cases was continuously dropping and this might be contributed to the active MMR vaccination campaigns carried out by the Lebanese health authorities targeting both lebanese and syrian children [20].
Salmonellosis is endemic in Lebanon and risk of outbreaks is always present [24]. Prevalence increases with poor hygiene and underdeveloped public health infrastructure [25]. Despite the large number of syrian refugees and the suboptimal health conditions, an annual average of 26 cases of salmonella infection was reported among this population between 2013 and 2016 as compared to an annual average number of 495 cases among the lebanese citizens (Figure 6). This may reflect suboptimal surveillance and reporting efforts. According to the CDC, it is estimated that for every one laboratory confirmed case of salmonella, there are 29 unconfirmed cases [26]. In Jordan it is believed that for each laboratory confirmed salmonella infection there are about 273 infected persons in the community [27]. The number of reported cases in Lebanon among nationals and syrians does not reflect the true incidence of the disease and more efforts are needed to monitor the spread of salmonella among syrian refugees and host community [12].
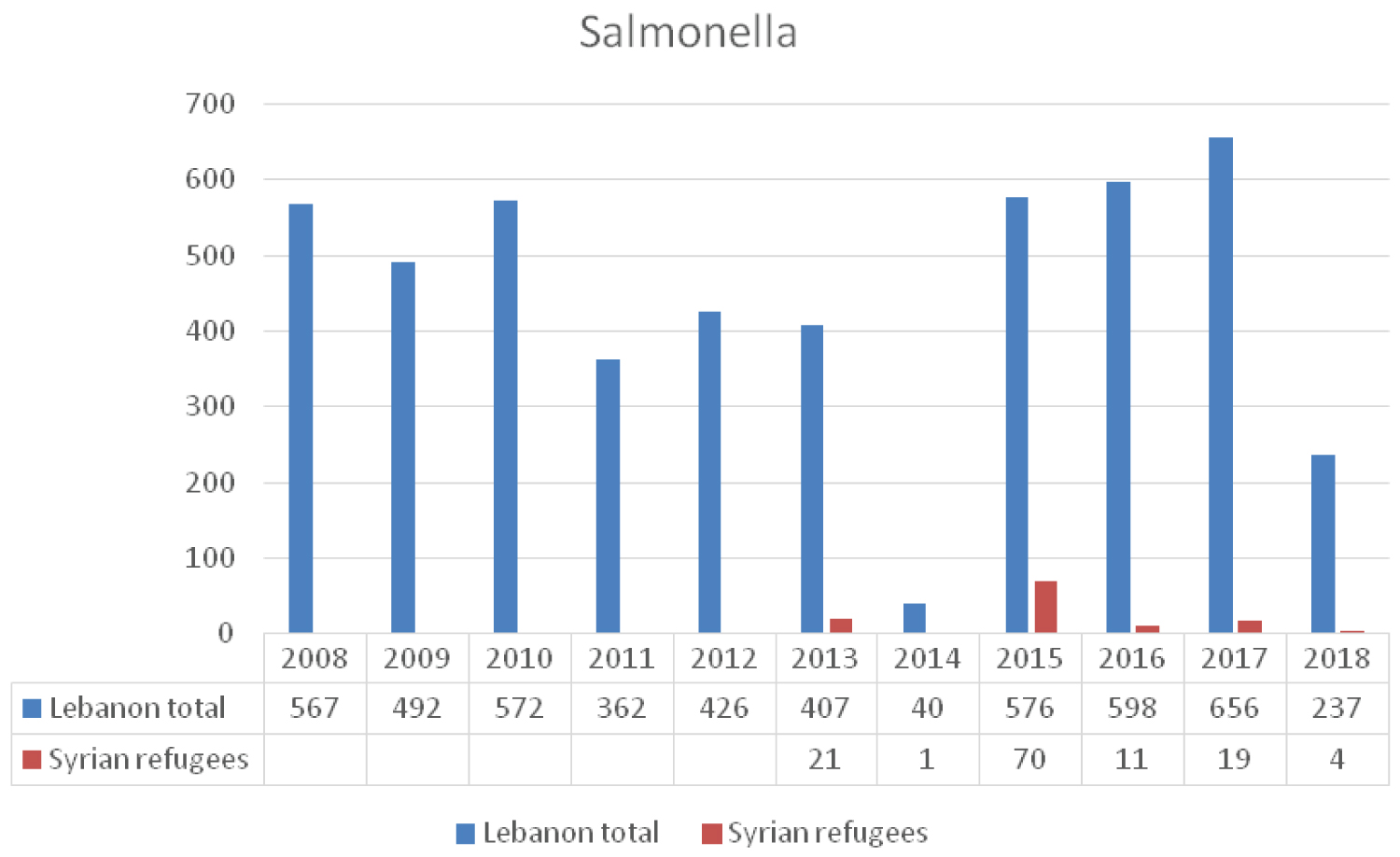 Figure 6: The reported cases of Salmonella to LMoPH-ESU from 2008 till 2016 including Lebanese nationals and Syrian refugees.
View Figure 6
Figure 6: The reported cases of Salmonella to LMoPH-ESU from 2008 till 2016 including Lebanese nationals and Syrian refugees.
View Figure 6
Brucellosis is an endemic zoonotic disease in the Middle East region with Syria being the country with the highest incidence in the world. Large numbers of animals are infected in Syria thereby causing significant human disease [28]. Although brucellosis is endemic in Lebanon and the occurrence of human disease is not uncommon [29], the last few years have witnessed a progressive increase in the incidence of reported human brucellosis cases (Figure 7) [18]. This increase might be attributed to high numbers of infected individuals among the Syrian refugees and to the uncontrolled and illegal animal migration (live stocks and cattle) [30] between the two countries. The literature cites two cases of brucellosis in Syrian refugees, one in Netherland and the other in Germany. The Netherland case was a 14-year-old boy who was diagnosed with neurobrucellosis, and brucella melitensis was identified in the cerebrospinal fluid. The German case [31] was a teenage woman migrating from syria. She became febrile postpartum and blood cultures revealed brucella melitensis.
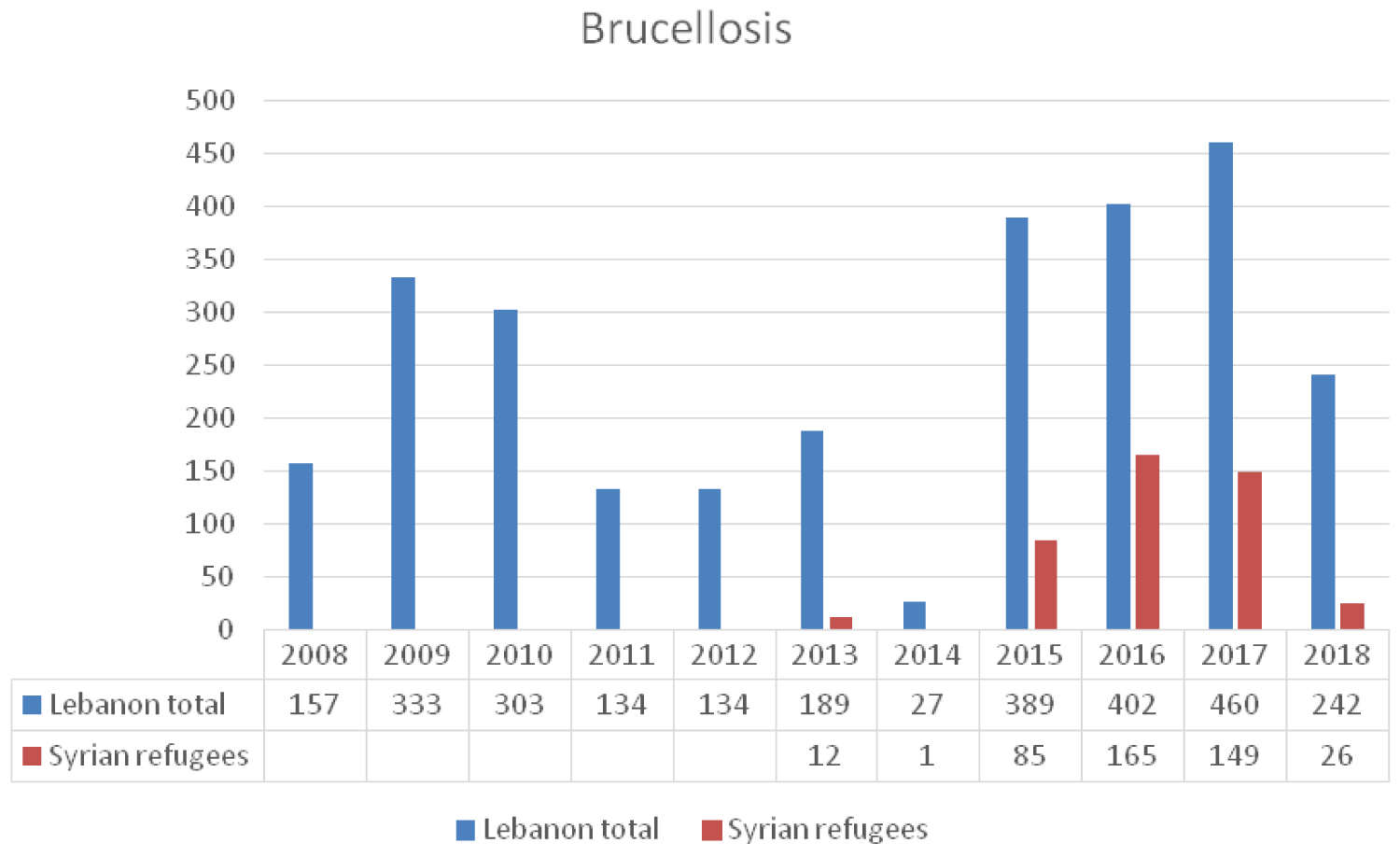 Figure 7: The reported cases of Brucella to LMoPH-ESU from 2008 till 2018 including Lebanese nationals and Syrian refugees.
View Figure 7
Figure 7: The reported cases of Brucella to LMoPH-ESU from 2008 till 2018 including Lebanese nationals and Syrian refugees.
View Figure 7
Conflicts and crises during war time contribute to migration and population displacements, and are often associated with increases in the risk of TB reaching up 20-foldss [31]. The NTP estimates that the total annual number of reported cases of active TB ranges between 500 and 700 cases, with a steep increase over the past few years [32] (Figure 8). According to the WHO Global TB reports, country profile section, Lebanon is not considered to be a high burden country regarding TB [33] where the estimated incidence rate is 16/100,000 per year and prevalence is 20/100,000 [32]. Despite this positive note, Lebanon remains at risk of an increasing TB burden; the country is located in a conflict area and a constant recipient of large numbers of displaced people. After a marked decrease in reported TB cases from 663 in 1999 to 375 cases in 2006, the number rose again reaching 689 in 2013, where the non-Lebanese constituted the majority of cases 348/341 including 106 syrian refugees. In 2012, the Lebanese accounted for around 52% of all reported cases of active TB cases with the Ethiopians ranking second (28%) followed by Syrians (16%). In the past 5 years this case distribution is shifting towards more non-Lebanese being affected exceeding 50% of total number of cases (Figure 8) [32]. The shift in the incidence of active TB cases from Lebanese to non-Lebanese mainly Ethiopians and Syrians may result in more individuals being affected and potential rise in the number of multidrug resistance TB (MDR-TB) cases.
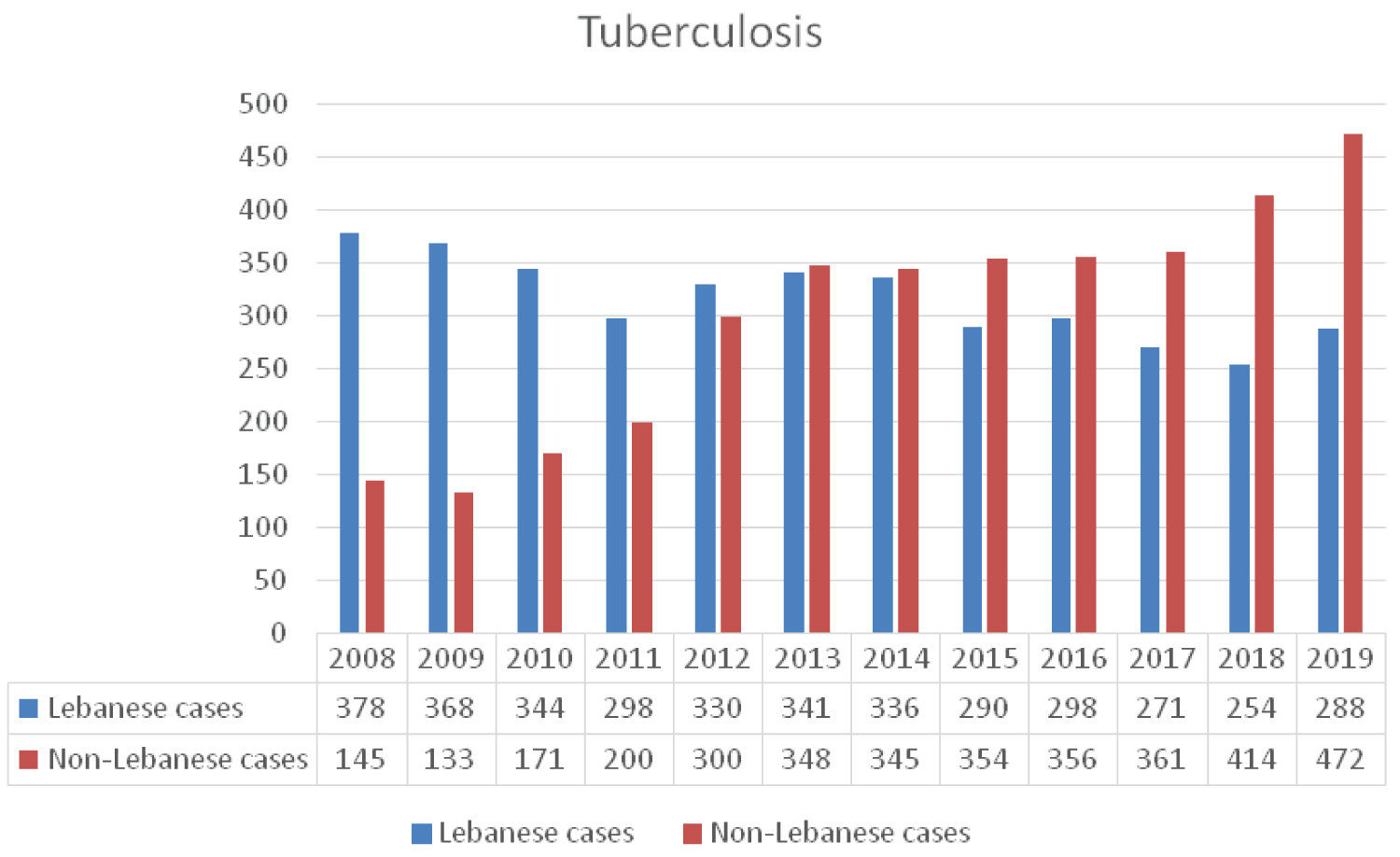 Figure 8: Trend of TB cases notification in national and non-national population by years from 2008 until 2019 According to NTP.
View Figure 8
Figure 8: Trend of TB cases notification in national and non-national population by years from 2008 until 2019 According to NTP.
View Figure 8
The NTP has a treatment success rate reaching 90% among Lebanese nationals. However, the treatment success rate remains below the desired levels among the non-Lebanese patients [18,32].
Two basic strategies are critical to the prevention and control of TB. The first priority is identifying and treating all people who have active TB. The second is contact screening and treating [34]. The Lebanese health authorities provide adequate and free treatment to all TB cases residing in the country including Syrian refugees [18,32,33,35]. Implementing this strategy to Syrian refugees faces several obstacles mainly due to security reasons, political affiliations and residence in difficult to reach rural areas. This remains a major concern especially if we consider the MDR-TB strains scattered across Syria reaching 62.5% [36]. In 2013, MDR-TB prevalence was 3% among Syrians in Lebanon as compared to 5% in Jordan [37].
An outbreak of leishmaniasis was reported among Syrian refugees residing in Lebanon in early 2013 [18]. Al Salem, et al. demonstrated that cutaneous leishmaniasis prevalence corresponds with the presence of refugee camps [38]. According to the LMoPH-ESU data, 1033 cases were reported between 2013 and 2014, with 96.6% of those affected being Syrian refugees [18]. The dense concentration of Syrian refugees along with minimal health care access in rural areas contributed to the increased incidences of leishmaniasis in Lebanon [19]. Risk factors for cutaneous leishmaniasis include poverty, malnourishment, population displacement, suppressed immunity, and poor housing [39]. Interventions by the LMPH led to containment of the disease and significant drop in leishmaniasis cases to 125, 110, 256, cases in 2015, 2017 and 2018 respectively, and near elimination of the disease in 2018 (Figure 9) [40].
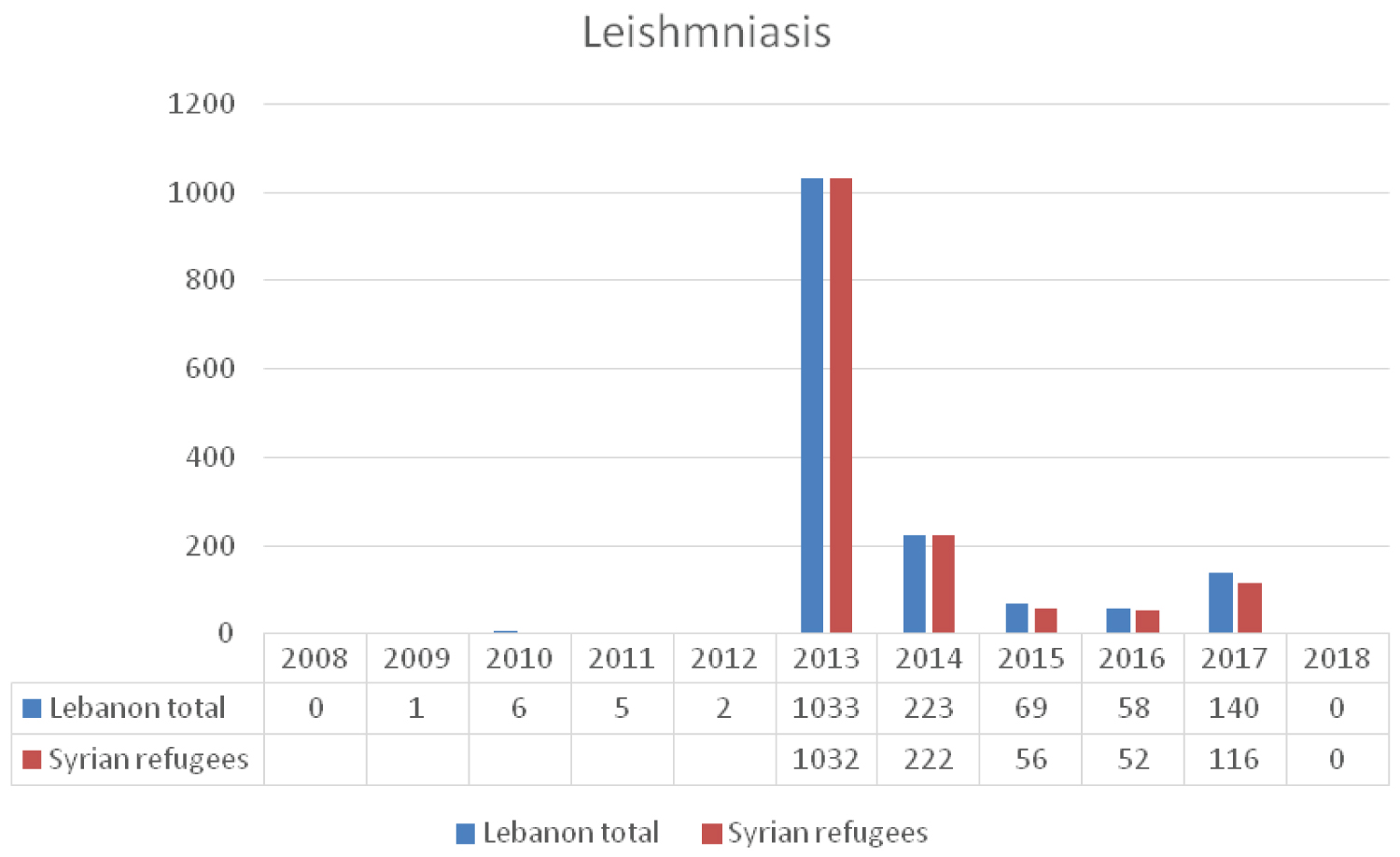 Figure 9: The reported cases of leishmania to the LMoPH-ESU form 2008 till 2018 including Lebanese nationals and Syrian refugees.
View Figure 9
Figure 9: The reported cases of leishmania to the LMoPH-ESU form 2008 till 2018 including Lebanese nationals and Syrian refugees.
View Figure 9
This drop in numbers of new cases is mainly attributed to the WHO support to the LMPH in developing a strategic response plan based on strengthening the surveillance system, establishing an updated national treatment protocol, putting in place a referral system of twelve treatment clinics across Lebanon and producing and disseminating public awareness and health education material [37].
Similarly, in Turkey the open border policy of turkish government allowed for large number of displaced Syrians to live in camps and according to the reports of Turkish Disaster and Emergency Management Presidency, the number of registered refugees residing in camps reached 22.088 in 2013 [41]. This resulted in emergence of leishmaniasis which peaked in 2013 and gradually decreased to three cases by 2015, while the registered number of Syrian refugees increase to 38.293 in the same year [40].
Data concerning syrians residing in Lebanon prior to the crisis was not available at the LMPH-ESU. While there is evidence of an increase in occurrence of several infectious diseases after the syrian influx to Lebanon, direct correlation cannot be established through the available data. This report presents information mainly based on literature reviews and surveillance data carried by LMPH-ESU, NTP and some actively involved non-governmental organizations; hence, different collection modalities, target population, sampling and selection methods were accumulated. Therefore, direct comparisons and recommendations for preventative measures cannot be definitive.
The influx of Syrians seeking refuge in Lebanon is associated with multiple unwanted effects. The infectious disease entities described in our manuscript can be categorized into:
1. Those that are considered as public health priorities for the host community and the refugees. This group is well represented by tuberculosis. The hosting government and the international community are committed to treat all affected whether a citizen or a refugee.
2. Those that are directly linked to the presence of Syrian refugees in Lebanon; namely leishmaniasis. It occurred almost exclusively among the Syrian refugees and can be attributed to the differences in rates of endemicity between Syria and Lebanon. Treating all affected mainly refugees aimed at containing the spread of infection among susceptible individuals and protecting the host community.
3. Those that may be indirectly affected by presence of large numbers of Syrian refugees such as hepatitis A, measles and mumps. They are vaccine preventable and the resulting outbreaks can be ascribed to several exacerbating local factors. Improving sanitary infrastructure and mass immunization campaigns to host and refugee community is essential in preventing major outbreaks.
4. Those that tend to be neglected among the Syrian refugee population residing in Lebanon. Chronic viral hepatitis B and C are good examples. The high cost of treatment prohibits local health authorities and the international community from any further action. The continued neglect of these entities may ultimately lead to major life threatening consequences among the refugees.
5. Those that are ignored due to either indolent clinical manifestations or long incubation period; namely brucellosis. The true consequences of neglect may manifest years later.
Several factors combined together including war, crisis, overcrowding, suboptimal sanitary infrastructure, and over stretched public health system may increase the incidence and facilitate the spread of infectious diseases, threatening the well-being of both host and refugee communities. Transmissible infectious diseases are of utmost significance since they have the potential to cross borders, alter local epidemiology, and result in significant health burden.