Pulmonary Kaposi sarcoma (KS) is a low-grade malignant neoplasm associated with human herpesvirus-8 (HHV-8), seen predominantly in immunocompromised hosts. Classically, diagnosis relies on the combination of clinical history, exclusion of alternative infectious and neoplastic etiologies, and histopathologic confirmation of proliferating spindle cells staining positive for HHV-8. Tissue-based diagnosis requires bronchoscopy with biopsy and is associated with procedural complications, such as bleeding and pneumothorax. In this case report, we describe a patient with a delayed diagnosis of pulmonary KS by classical invasive modalities, highlight rapid and non-invasive diagnostic testing through retrospective application of metagenomic sequencing, and illustrate the complex clinical course which ultimately led to the patient's demise.
A 40-year-old African-American male with human immunodeficiency virus (HIV) with CD4 count 11 cells/mL and an undetectable viral load on antiretroviral therapy and recent Cryptococcus neoformans meningitis on suppressive fluconazole therapy was admitted to the intensive care unit (ICU) with two weeks of progressive exertional dyspnea. He was found to have acute hypoxemic respiratory failure requiring mechanical ventilation. Computed tomography (CT) demonstrated diffuse bilateral patchy airspace disease consistent with acute respiratory distress syndrome (ARDS) (Figure 1), in addition to numerous lytic lesions of the sternum, vertebral bodies, and iliac crest. The patient was started on empiric broad-spectrum antimicrobial therapy, including sulfamethoxazole-trimethoprim for possible Pneumocystis jirovecii pneumonia, the leading differential diagnosis at the time given his clinical presentation and immunocompromised status. Bronchoalveolar lavage (BAL) sampling was negative for bacterial pathogens, fungal elements (including Pneumocystis spp.), and acid-fast bacilli. Biopsies were not performed due to the severity of his acute illness. He improved with supportive care and was extubated on hospital day four. The patient underwent fluoroscopic-guided iliac crest bone biopsy on hospital day ten for incidentally discovered lytic lesions on imaging, which revealed spindle cell proliferation staining positive for human herpesvirus-8 (HHV-8) (Figure 2A and Figure 2B), thereby establishing the diagnosis of Kaposi sarcoma (KS). He was started on outpatient doxorubicin therapy following discharge.
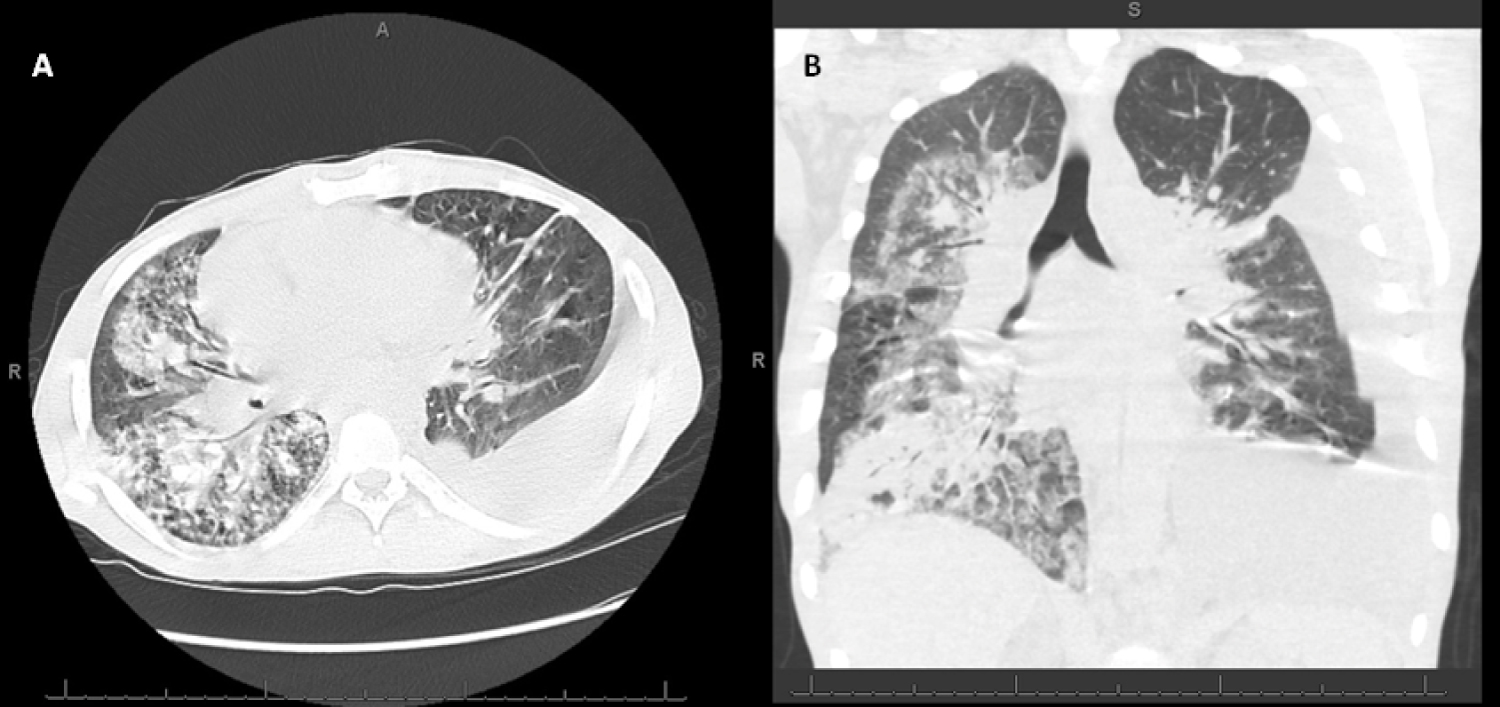 Figure 1: Chest CT at Admission (A) Transverse (cross-sectional) and; B) Coronal cuts demonstrating extensive patchy bilateral consolidations, ground glass opacities, and interlobular septal thickening.
View Figure 1
Figure 1: Chest CT at Admission (A) Transverse (cross-sectional) and; B) Coronal cuts demonstrating extensive patchy bilateral consolidations, ground glass opacities, and interlobular septal thickening.
View Figure 1
 Figure 2: Histopathology from Bone Marrow and Endobronchial Biopsies. (A) Bone marrow biopsy with positive immunostain for HHV-8; (B) Bone marrow biopsy with positive immunostain for the vascular marker ETS-related gene (ERG); (C) Endobronchial biopsy hematoxylin and eosin (H&E) stain with atypical spindle cells arranged in fascicles and slit-like spaces associated with scattered interstitial inflammatory cells with perineural invasion; (D) Endobronchial biopsy with positive immunostain for HHV-8.
View Figure 2
Figure 2: Histopathology from Bone Marrow and Endobronchial Biopsies. (A) Bone marrow biopsy with positive immunostain for HHV-8; (B) Bone marrow biopsy with positive immunostain for the vascular marker ETS-related gene (ERG); (C) Endobronchial biopsy hematoxylin and eosin (H&E) stain with atypical spindle cells arranged in fascicles and slit-like spaces associated with scattered interstitial inflammatory cells with perineural invasion; (D) Endobronchial biopsy with positive immunostain for HHV-8.
View Figure 2
Over the ensuing two months, the patient endorsed a persistent productive cough with recurrent expectoration of white/yellow fibrinous material that, when manually unfolded, revealed castings of his tracheobronchial tree (Figure 3). Due to concern for pulmonary KS, he underwent outpatient bronchoscopy approximately ten weeks following initial intubation, which revealed diffuse mucosal nodular lesions and fibrinous mucus. Histopathology from endobronchial biopsies returned with squamous cell metaplasia and spindle cell proliferation staining positive for HHV-8 (Figure 2C and Figure 2D). His bronchial casts demonstrated elevated triglyceride level (885 mg/dL) concerning for intrathoracic lymphatic invasion; subsequent lymphangiography did not demonstrate passage of lipiodol contrast into the thoracic ducts (Figure 4). Due to concerns for disease progression on doxorubicin, the patient's chemotherapy regimen was transitioned to paclitaxel.
 Figure 3: Bronchial Cast. Expectorated by patient and unfolded to reveal branching divisions of tracheobronchial tree, consistent with plastic bronchitis.
View Figure 3
Figure 3: Bronchial Cast. Expectorated by patient and unfolded to reveal branching divisions of tracheobronchial tree, consistent with plastic bronchitis.
View Figure 3
 Figure 4: Lymphangiography. No passage or progression of lipiodol contrast injected into left groin beyond the L3 thoracic duct after three hours; unable to evaluate chest lymphatic structures.
View Figure 4
Figure 4: Lymphangiography. No passage or progression of lipiodol contrast injected into left groin beyond the L3 thoracic duct after three hours; unable to evaluate chest lymphatic structures.
View Figure 4
Unfortunately, several weeks later, he suffered an out-of-hospital cardiac arrest with return of spontaneous circulation, presumed secondary to acute hypoxemic respiratory failure, with resultant multisystem organ failure. His care was transitioned to comfort measures. No autopsy was performed.
During the patient's initial ICU course, he was enrolled into an observational cohort study of mechanically ventilated patients with acute respiratory failure conducted across several ICUs within our health system. From enrolled patients, we collected non-invasive endotracheal aspirate (ETA) and plasma samples within 72 hours of intubation for study of the respiratory microbiome communities [1]. We performed metagenomic sequencing of microbial deoxyribonucleic acid (DNA) from ETA samples with the Nanopore MiNION platform (Oxford Nanopore Technologies, Inc.) [2]. Due to high quantities of human host DNA contaminating ETA samples in critically-ill patients, we applied a human DNA depletion pre-processing technique involving saponin-based lysis of human cells and nuclease-induced digestion of extracellular DNA to target bacterial, viral, and fungal DNA enrichment [3]. In our patient, we also performed post-hoc cytologic examination and immunohistochemistry staining for HHV-8 and cytomegalovirus (CMV) antigens from an aliquot of raw (pre-processed) ETA sample. A sample of 500 μL of plasma was used to perform the Karius Test (Karius, Inc.), an agnostic metagenomic sequencing of microbial cell-free DNA for the rapid detection of bacterial, viral, and fungal pathogens in the systemic circulation [4].
Metagenomic sequencing of the ETA sample using the Nanopore MiNION platform yielded a total of 160,519 microbial DNA sequences (reads), of which 121,866 sequences (76%) aligned with Rothia mucilaginosa and the remainder with Streptococcus spp., which are all typical members of the oropharyngeal microbiome community (Figure 5). There was no P. jiroveci DNA detected, which was the leading diagnostic consideration at the time of the clinical encounter. Notably, Nanopore sequencing did not detect any viral DNA (including HHV-8 DNA), as the pre-processing method with host DNA depletion removed viral DNA from the ETA sample. Immunohistochemistry staining of ETA cells for HHV-8 or CMV was also negative.
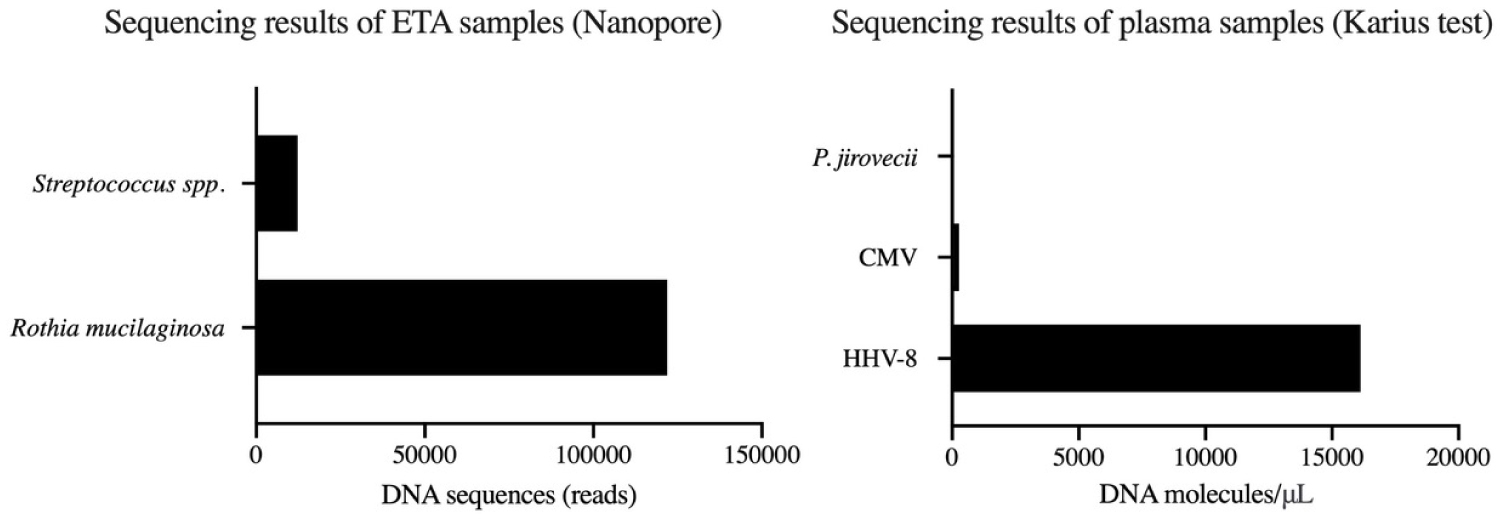 Figure 5: Metagenomic Sequencing. (A) ETA. Nanopore sampling with profile representative of oropharyngeal microbiome community; (B) Plasma. The Karius Test using plasma cell-free DNA, which identified a high load of HHV-8 DNA and a small load of CMV DNA. Data not publically available.
View Figure 5
Figure 5: Metagenomic Sequencing. (A) ETA. Nanopore sampling with profile representative of oropharyngeal microbiome community; (B) Plasma. The Karius Test using plasma cell-free DNA, which identified a high load of HHV-8 DNA and a small load of CMV DNA. Data not publically available.
View Figure 5
Metagenomic sequencing of plasma cell-free DNA with the Karius Test revealed an extremely high HHV-8 DNA load (16,123 molecules/μL) (Figure 5). A low CMV DNA load (273 molecules/μL) was also detected. There was no evidence of bacterial or fungal DNA, further supporting the absence of a co-infection at the time of the initial clinical encounter.
HHV-8 is a double-stranded DNA virus involved in the pathogenesis of several diseases, most notably, KS. HHV-8-related disease manifests almost exclusively in immunocompromised hosts-often in patients with advanced HIV disease or acquired immune deficiency syndrome (AIDS). KS is a low-grade angiolymphoproliferative mesenchymal tumor associated with infection of spindle cells, initially with a benign, reactive polyclonal cellular response that eventually undergoes malignant transformation [5,6]. Patients with KS generally have mucocutaneous and visceral organ involvement, although these features may be absent in 5-23% of patients with symptomatic pulmonary involvement [7]. Pulmonary KS manifests with non-specific symptoms of fever, dyspnea, productive cough, wheezing, and chest pain [8]. This may progress to acute hypoxemic respiratory failure. Plastic bronchitis, described as the expectoration of bronchial casts, has been associated with abnormal thoracic lymphatic drainage and flow [9].
The diagnosis of pulmonary KS can be challenging. It is typically made through the combination of clinical history, histopathology, and exclusion of other infectious or neoplastic processes [10]. Radiographically, pulmonary KS has a diverse array of non-specific findings given its possible involvement of the tracheobronchial tree, parenchyma, pleural, and lymphatic system. Chest radiography may demonstrate middle or lower lung field reticular opacities, parenchymal nodules, or consolidation [11]. CT chest may reveal prominent interstitial thickening, interlobular septal thickening, ground glass opacities, parenchymal nodules with ill-defined borders, or pleural effusions [8]. Bronchoscopy with biopsy is typically pursued for tissue confirmation. Endobronchial lesions are identified in 45-73% of cases, with a typical appearance of maculopapular, erythematous, or violaceous lesions that is most commonly discrete in the trachea but may be more diffusely confluent in distal airways [11,12]. Generally, endobronchial or transbronchial biopsies only have modest diagnostic yield and carry high risk of bleeding from surrounding inflammation and angioinvasion. The presence of spindle cell proliferation on histopathology with positive immunostaining for HHV-8 is both pathognomonic and diagnostic [10,11,13].
There are many challenges associated with obtaining tissue histopathology: The need for a skilled proceduralist, the procedural sedation accompanied by adverse reactions, the considerable procedural risks of airway bleeding and pneumothorax, the pathology processing time, and the potential for inadequate sampling and/or false negatives, among others. As a result, there have been recent efforts to shift toward non-invasive measures for diagnosis. The emergence of molecular detection modalities [14,15] have allowed for the utilization of HHV-8 polymerase chain reaction (PCR) from BAL fluid [16], while bead-based serologic testing have led to quantitative determination of antibody levels related to HHV-8 replication and disease risk [17].
In our patient, the use of the Karius Test (Karius, Inc.), a plasma metagenomics platform, assisted in the identification of substantial quantities of plasma cell-free HHV-8 DNA on the same day as intubation and mechanical ventilation, and took place approximately ten days prior to undergoing invasive bone marrow biopsy. Additionally, metagenomic sequencing of the patient's ETA detected the presence of an oropharyngeal microbiome community without demonstration of P. jirovecii or other bacterial or fungal pathogens. In this case, these non-invasive modalities may have assisted in the earlier diagnosis of KS and allowed clinicians to have narrow antimicrobial therapy, decrease harmful adverse effects of unnecessary antimicrobial use, avoid unnecessary invasive testing, and minimize procedural sedation.
The diagnosis of pulmonary KS has, historically, relied on invasive tissue sampling for histopathology, in addition to a clinical history and the exclusion of other processes. Novel non-invasive metagenomic sequencing platforms have been recently developed to aid in the rapid detection of bacterial, viral, and fungal elements while providing diagnostic certainty when encountering clinical challenges. In this patient's case, the ability to rapidly diagnose KS and exclude other infectious etiologies carries future promise of utilizing non-invasive testing to avoid unnecessary procedures and medications. As more refined diagnostic approaches emerge, additional research is necessary to define their diagnostic validity and practical utility in clinical care.
R.H.Z., G.D.K., and C.M.H. designed the study. R.H.Z., P.A.Z, and J.M.B. prepared the initial draft. V.D.N. provided sequencing data. D.M.C., M.S.L, and H.T.B. provided histopathology images. All authors contributed to manuscript revisions.
G.D.K. has received research funding from Karius, Inc. All other authors report no conflicts of interest.