Shiga toxin 2 (Stx2) is a potent toxin produced by shiga toxin-producing E. coli (STEC) that is commonly linked to development of hemolytic uremic syndrome (HUS). An extremely rare complication of shiga toxin associated (STEC) hemolytic uremic syndrome is concomitant infection with Clostridium septicum. The majority of pediatric patients with HUS complicated by C. septicum infection had complications including CNS involvement, severe renal insult often requiring hemodialysis, and myonecrosis warranting invasive surgical intervention, and prolonged administration of multiple antimicrobial agents. To date, there are 12 reported pediatric cases of HUS with concomitant C. septicum infection. Of the 12 reported cases, only 6 survived - all of whom warranted either surgical intervention, hemodialysis and prolonged antibiotic therapy, or both. We report a case of a 4-year-old female who presented with bloody diarrhea, emesis and fever with STEC HUS complicated by C. septicum bacteremia who was successfully treated with a short course of metronidazole without hemodialysis or surgical intervention.
HUS, C. septicum, Bacteremia, Shiga toxin
HUS: Hemolytic Uremic Syndrome; STEC: Shiga Toxin-producing Escherichia coli; Stx2: Shiga toxin 2; MIC: Minimum Inhibitory Concentration
A 4-year-old Caucasian female with developmental delay and cerebral palsy presented with profuse bloody diarrhea, abdominal pain and non-bilious, non-bloody emesis one day prior to admission. There was no identified potential exposure or sick contact other than a class zoo trip without her two weeks prior to presentation. Nucleic acid amplification testing for common enteric pathogens was performed on admission and Stx2 was detected. Leukocytosis (WBC 20.8 × 109/L) with neutrophil predominance (ANC 15.76 × 109/L) was identified. Other routine blood count and chemistry tests were within normal ranges. She was initially managed with intravenous fluid replacement.
On hospital day 2, an isolated fever of (38.4 °C) was recognized and resolved without intervention, but day 3 of hospitalization included intermittent bouts of fever (Tmax = 39.6 °C), leukocytosis (WBC 30.3 × 109/L) and neutrophilia (ANC 23.51 × 109/L) had increased, haptoglobin was low (< 30 mg/dL) and lactate dehydrogenase was elevated (430 U/L). Blood and urine cultures were obtained, and ceftriaxone 50 mg/kg was empirically started for a possible urinary tract infection based on urinalysis which showed leukocyturia of 51-100/HPF and large leukocyte esterase. Three hours following initial ceftriaxone administration, the patient's condition worsened with increased fever (40.1 °C), tachycardia (139/min), tachypnea (30/min) and hypotension (94/50 mmHg). Hemoglobin and platelets had fallen below the reference range (8.6 g/dL & 81 × 109/L, respectively), and lactate dehydrogenase had markedly increased (1108 U/L). The patient developed acute hemolytic anemia and acute kidney injury consistent with hemolytic uremic syndrome (HUS). Ceftriaxone was held due to concern that it could potentially advance hemolysis and worsen renal function.
Serial labs showed nadir hemoglobin of 3.51 g/dL and a platelet count of 6.1 × 109/L on hospital day 4. The patient developed oliguric acute kidney injury with peak serum creatinine of 2.1 mg/dL on the same day. These findings further supported the diagnosis of HUS with its associated acute hemolysis and deteriorating renal function. Urine culture grew 20,000-80,000 CFU/ml of Escherichia coli. After 28 hours of incubation, the anaerobic blood culture bottle became positive with boxcar-shaped gram-positive bacilli (Figure 1), which was later identified as Clostridium septicum by MALDI-TOF mass spectrometry. The patient received multiple blood transfusions on hospital days 6, 11, 13 and received platelet transfusion on hospital day 11.
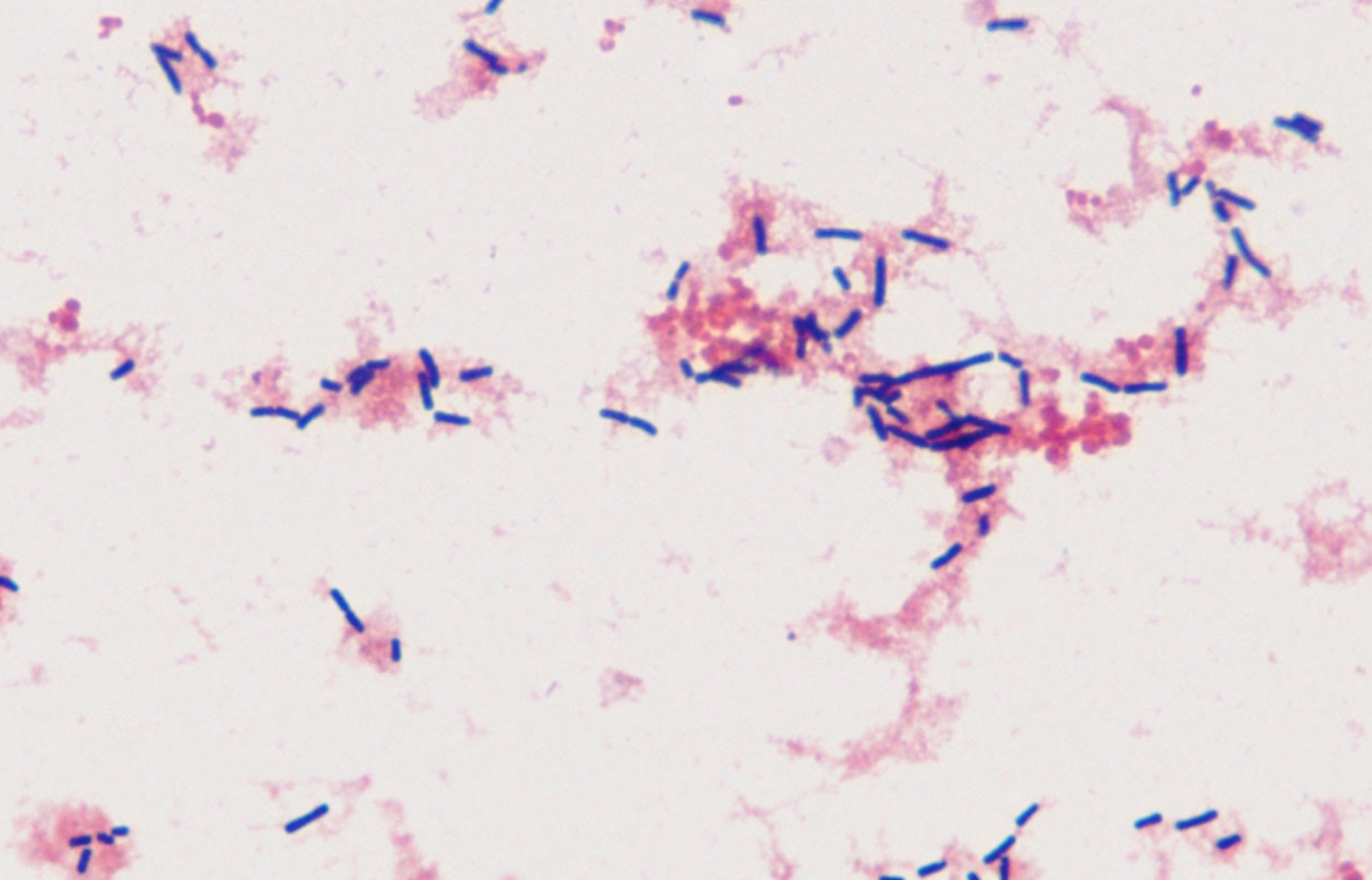 Figure 1: Box-car shaped Gram-positive bacilli (Clostridium septicum) seen on Gram stain of the patient's blood culture under 1000x magnification.
View Figure 1
Figure 1: Box-car shaped Gram-positive bacilli (Clostridium septicum) seen on Gram stain of the patient's blood culture under 1000x magnification.
View Figure 1
The patient continued to have intermittent bloody diarrhea and tarry stools during her course of admission in the pediatric intensive care unit. Stooling returned to normal baseline on hospital day 9. She completed 7-day course of metronidazole for the C. septicum bacteremia per susceptibility result (MIC 0.125 to metronidazole). She was discharged on hospital day 15, and at discharge her lab results were normalizing with serum creatinine 0.57 mg/dL, hemoglobin 7.8 g/dL, and a platelet count 179 × 109/L.
C. septicum is a strictly anaerobic, gram-positive, alpha-toxin-producing bacillus that causes atraumatic, rapidly-progressive gas gangrene that is often precipitated by bacteremia from gut translocation secondary to compromised gastrointestinal mucosal surface from inflammation, neutropenic colitis, leukemia or diabetes [1,2]. Only about 2% of humans carry this bacterium in their gut microbiota [3]. Anaerobic blood culture is an absolute requirement if C. septicum is to be isolated as a cause of bacteremia. The etiology of the bacteremia is thought to be due to a compromised blood-gut barrier during colitis [1]. Twelve cases of E. coli HUS complicated by C. septicum bacteremia have been reported to date. Common presenting symptoms include bloody diarrhea, abdominal pain and fever. Clinical courses and treatment responses have been variable (Table 1) [2,4-13].
Table 1: Review of reported cases of HUS with concomitant C. septicum infection. View Table 1
Many of these pediatric cases describe involvement of the CNS and soft tissues warranting surgical interventions in addition to antibiotics [5-7,9-12]. Of the patients with CNS involvement, only two patients survived despite prompt antibiotic therapy, hemodialysis, and neurosurgical intervention [6,9]. One patient received penicillin and metronidazole with emergent ventriculostomy but succumbed 6 hours after admission [10]. All three patients who neither received prompt medical nor surgical therapy did not survive [5,7,11]. One patient described without CNS involvement developed ecchymotic necrosis of right arm survived after antibiotic therapy and amputation of the ischemic extremity [2].
Iddings, et al. describes a 5-year-old male with newly diagnosed type 1 diabetes mellitus who initially presented in DKA and loose non-bloody stools who developed HUS from Shiga-like toxin producing E. coli O157:H7 with concomitant C. septicum bacteremia [4]. He subsequently developed intestinal perforation warranting exploratory laparotomy, sigmoid colon resection and diverting loop ostomy placement. He developed high-output chronic kidney disease from HUS and needed hemodialysis. The patient's course was further complicated by polymicrobial peritonitis. He was successfully treated with 21-day course of antibiotics.
The current case is the third pediatric uncomplicated case of E. coli HUS with C. septicum bacteremia that has been reported. Similar to the current case, the other two cases were successfully managed with antibiotics and not surgical intervention, but temporary hemodialysis was required in the other two cases [5]. The first was a case of a 4-year-old female exposed to a petting zoo who presented with fever, bloody diarrhea and abdominal pain. She was treated with metronidazole, clindamycin and meropenem for 14 days. The other case was of a 6-year-old female without known exposure who presented similarly and who received 7 days of penicillin. Both patients survived without reported sequelae. Of the reported cases of E. coli HUS complicated by C. septicum bacteremia, half (7/13) of the patient died, and half (3/6) survivors required surgical intervention with most (5/6) requiring hemodialysis. To our knowledge, the current case is the only reported case of E. coli HUS complicated by C. septicum bacteremia to be successfully treated with only a short course of metronidazole and no hemodialysis or surgical intervention.
This article presents a rare case of Stx2-mediated hemolytic uremic syndrome with Clostridium septicum bacteremia that was successfully treated with short course antibiotic without necessitating hemodialysis or surgical intervention.
All authors equally contributed to the work of this manuscript.
Authors declare no conflict of interest.