Hepatitis B infection continues to be a serious global health problem with about 2 billion people infected worldwide, many of these in sub-Saharan Africa.
Records of Hepatitis B surface and envelope antigen test results in adults attending the outpatient units and/or admitted in Federal Teaching Hospital, Gombe between May 2000 and May 2015 were retrieved and analyzed.
Hepatitis B surface antigen was tested for in 22,862 adults and children; 20375 (89.1%) were adults and 2487 (10.9%) were children.
Adult Males were 11420 (56.0%) and females 8955 (44.0%). 4796 (23.5%) of tested adults were 19-25years; 11634 (57.1%) 26-45 years; 2074 (10.2%) 46-55 years; 5.5% (1112) and 759 (5.4%) > 65years.
4015/20375 (19.7%) were positive for Hepatitis B surface antigen. 2883/4015 (71.8%) males and 1132 (28.2%) females were Hepatitis B positive. 887/4015 (18.5%) between 19-25 years, 2533 (21.8%) among 26-45 year old individuals; 397 (19.1%); 136 (12.2%) and 62 (8.2%) of 46-55 year old, 56-65 year old and > 65year old individuals screened were Hepatitis B positive.
1361/4015 (34.0%) of HBsAg seropositive individuals were tested for HBe antigen. Males were 954 (69.4%) and females 407 (30.6%). HB e antigen was positive in 321/1361 (23.6%) of HBsAg carriers; 238 (74.1%) males and 83 (25.9%) females. 133 (41.4%); 165 (51.4%); 17 (5.3%); 4 (1.2%) and 2 (0.6) of HB e sero-positivity were among age groups 19-25, 26-45, 46-55, 56-65 and > 65 year old individuals respectively. 95/226 (42.0%); 126/600 (21.0%); 13/100 (13.0%); 3/23 (13.0%) and 1/5 (20.0%) of males aged 19-25; 26-45; 46-55; 56-65; and > 65 years had HB e antigen respectively. Among females 38/116 (44.2%) aged 19-25; 39/241 (16.2%) 26-45; 4/44 (9.1%) 46-55; 1/5 (20.0%) 56-65 and 1/1 (100%) had HBe antigen.
Prevalence of Hepatitis B surface antigen is high in our facility and overall prevalence of Hepatitis B e antigen of 23% is an under estimation as only a third of Hepatitis B surface antigen carriers were tested. Implication for treatment is significant.
Viral hepatitis is a major public health challenge and the substantial global burden of hepatitis B virus (HBV) infection is increasingly recognised [1]. Hepatitis B virus (HBV) infection results in substantial human morbidity and mortality, predominantly through the consequences of chronic infection [2].
Recent estimates suggest that HBV infection caused 887000 deaths in 2015 [1], placing HBV in the top 20 causes of human mortality [3]. WHO estimates that in 2015, 257 million persons, or 3.5% of the population, were living with chronic HBV infection in the world. The African and Western Pacific regions accounted for 68% of that infected [1]. Africa is considered endemic for hepatitis B surface antigen where 6.1% of the general population is chronic carriers [4,5].
Access to affordable hepatitis testing is limited. Only 9% of people with viral hepatitis have been diagnosed [1]. While several risk factors for Hepatitis B infection are known [6], the low coverage of an effective vaccine that is widely available in Nigeria [7,8], and the limited access to affordable Hepatitis testing pose [1,4] significant public challenge to the country. Low level of community participation, inadequate cold chain infrastructure and poor funding for routine immunization amongst other factors remain barriers to improving immunization coverage in Nigeria [7,8].
Infection with HBV may present as either hepatitis B "e-antigen" (HBeAg) positive or -negative disease. Hepatitis B "e-antigen" is seen in many HBeAg-positive children and young adults, particularly among those infected at birth [1,2]. In persons with CHB, a positive HBeAg result usually indicates the presence of active HBV replication and high infectivity. Clinically, HBeAg is an index of viral replication, infectivity, inflammation, severity of disease and response to antiviral therapy [9].
Musa, et al. [10] in a systemic review and meta-analysis of the prevalence of Hepatitis B Virus infection in Adult Nigerians between 2000-2013 established a pooled prevalence of 14%. A National survey of Hepatitis B in 2016 in the general population by the Federal Ministry of Health showed prevalence of 11% with states in the north of the country having higher prevalence of Hepatitis B than the southern states [6]. In both studies [6,10], the status of HBe Ag were not reported however these were bold efforts at establishing correct estimate and true burden of Hepatitis B virus infection in Nigeria.
Recent Nigeria studies from Jalingo [11], Kaduna [12] Sokoto [13], Kogi [14] and Makurdi [15] showed the prevalence of Hepatitis B Virus of 19.2%, 14%, 63%, 25% and 39% in various adult subpopulations of hospital patients respectively. Hepatitis B testing methods were different.
HBeAg is an important predictor of HBV related liver disease and a key determinant for treatment of hepatitis b [9,16].
Earlier reports [17-21] and recent studies [22-26] on the prevalence of HBeAg in adults with Hepatitis b infection in Nigeria had small sample sizes, short study duration and limited age and sex distribution.
The objective of this study is to report the carriage of Hepatitis B Envelope antigen in Adult chronic carriers of Hepatitis B virus in a teaching hospital in Nigeria from 2000-2015.
Gombe is the capital of Gombe state. Gombe state is one of the six states that comprise North East Geopolitical zone in the country and one of the geopolitical zones with the highest levels of poverty and worse maternal and child health indices [8,27].
This study was conducted in Federal Teaching Hospital Gombe, a 500 bed hospital serving Gombe and neighboring states. The Federal Teaching Hospital, Gombe (FTHG) started providing services in the year 2000. It has emerged as a Centre for treatment, teaching and research in the sub region with large patient referrals from the neighbouring states of Borno, Yobe, Adamawa and Bauchi.
All adults who presented to the medical out-patient department and those that were admitted, irrespective of their HIV and/or Hepatitis C viral status, and who had Hepatitis B and/or Hepatitis B envelope antigen test from 2000 to 2015.
All adults were tested using the Hospital standard for Hepatitis B surface antigen test strip. The ACON HBsAg (ACON Laboratories, Incorporated San Diego, California, USA) is a rapid one step test for the qualitative detection of Hepatitis B surface Antigen and Hepatitis B envelope antigen in serum or plasma. The HBsAg test strip has a relative sensitivity, greater than 99.8% and specificity of 99.7%.
The ACON HBeAg (ACON Laboratories, Incorporated San Diego, California, USA).The HBeAg EIA Test Kit is a one-step enzyme immunoassay for the qualitative detection of Hepatitis B Envelope Antigen (HBeAg) in human serum or plasma.
The ACON HBsAg One Step Test is a qualitative, solid phase, two site sandwich immunoassay for the detection of Hepatitis B surface Antigen (HBsAg) and envelope antigen in serum or plasma. The membrane is pre-coated with anti-HBsAg antibodies on the test line region and anti-mouse antibodies on the control region. During testing the serum or plasma samples reacts with dye conjugate (mouse anti-HBsAg antibody-colloidal gold conjugate) which has pre-coated in the test strip. The mixture migrated upwards on the membrane chromatographically by capillary action to react with anti-HBsAg antibodies on the membrane and generates a red line. Presence of this red line indicates a positive result, while its absence indicates a negative result. Regardless of the presence of HBsAg as the mixtures continues to migrate across the membrane to the immobilized goat anti-mouse region, a red line at the control region will always appear. The presence of this red line serves as verification for sufficient sample volume and proper flow as a control for the reagents [28].
HBeAg in the sample first bound to anti-HBe antibodies coated on the micro-particles, and then the bound HBeAg was detected upon addition of anti-HBe antibodies conjugated to alkaline phosphatase. The HBeAg levels were evaluated using ratios of sample to cut-off values (S/CO), and HBeAg positivity was suggested if the S/CO was ≥ 1.0. Verification of test results was carried out by randomly retesting 5% of the specimens using the same kit.
Records of Hepatitis B surface and envelope antigen results of adults in Federal Teaching Hospital, Gombe between 2000 and 2015 were retrieved. Variables analysed included age, sex, year, month, and hepatitis B surface and envelope antigen.
All records were imputed into EPI info Version 3.2 and analysed.
During the study period 676,673 adults were consulted in outpatient and 75,528 were admitted in the federal teaching hospital Gombe between 2000 and 2015 (Figure 1).
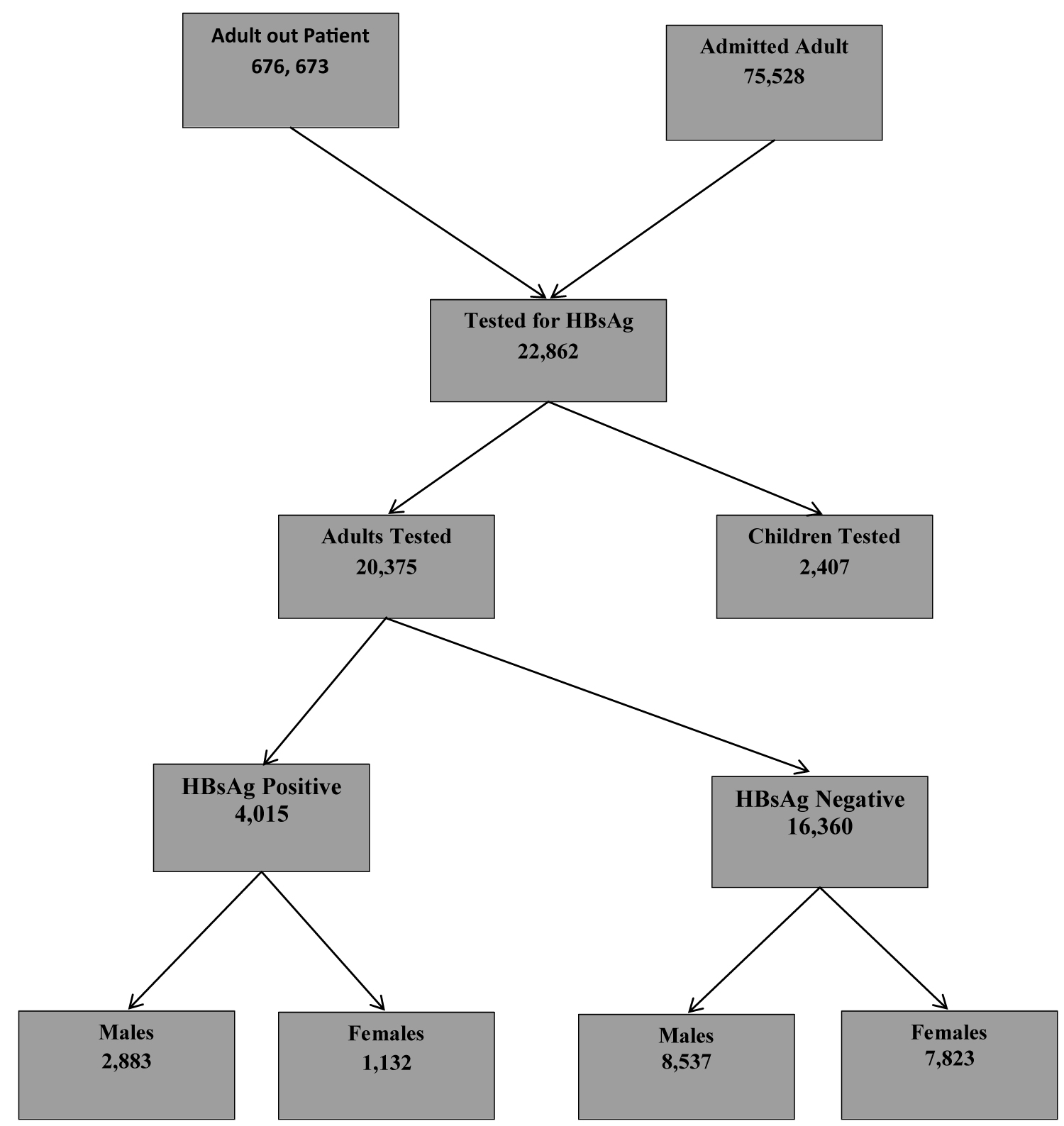 Figure 1: Adults tested for HBsAg from 2000-2015 in Federal Teaching Hospital Gombe, North East.
View Figure 1
Figure 1: Adults tested for HBsAg from 2000-2015 in Federal Teaching Hospital Gombe, North East.
View Figure 1
20375 adults were tested for HBsAg. Males constituted 56.0% (11420) and 44.0% (8955) were females. In both adult males and females, the age group 26-45 years was the predominant age tested for HB infection and those > 65 years were the least (Table 1).
Table 1: Age and Sex distribution of adults screened for Hepatitis B. View Table 1
The prevalence of Hepatitis b virus infection was 19.7%. Males were twice as likely as females to be infected with hepatitis B and were statistically significant and this was so across all age groups except age groups 56-65 and above 65 years (p < 0.001) (Table 2). Hepatitis B prevalence was highest (28.6%) in younger male age group (19-25 years) compared to 15.6% in the female age group (26-45 years) Table 2 (Figure 2).
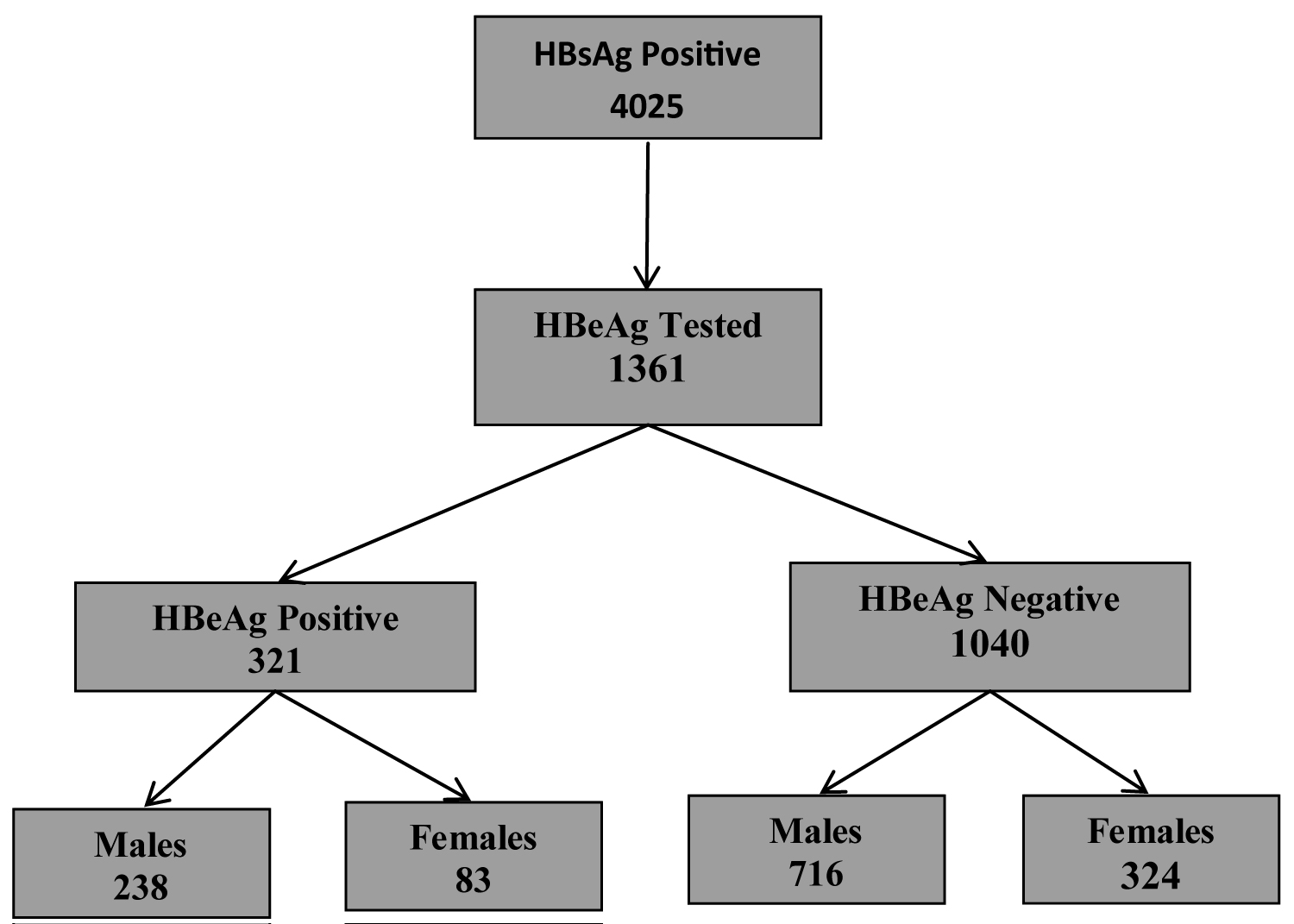 Figure 2: HBeAg positive adult carriers of HBsAg.
View Figure 2
Figure 2: HBeAg positive adult carriers of HBsAg.
View Figure 2
Table 2: Age and sex distribution of test results of Adults screened for HBsAg. View Table 2
Table 3 showed that of the 4015 adult males and females who tested positive for HBsAg, (33.9%) 1361 were tested for HBeAg and 23.6% (321/1361) were reactive for the presence of the envelope antigen. The sex distribution was not significant.
Table 3: Age and Sex distribution of adults HBsAg + and tested for HBeAg. View Table 3
About a fifth of adults who were HBsAg carriers tested positive for Hepatitis B envelope antigen. More male than female HBsAg carriers tested positive for the envelope antigen though this was not statistically significant (Table 4). Age distribution for HBeAg was statistically significant; Hepatitis Be antigen was highest in the 19-25 year age chronic carriers of HBV and continuously declined until the age > 65 years where there was a rise in its prevalence .There was no statistical significance in the distribution of HBeAg when disaggregated by age group and sex.
Table 4: Age and Sex distribution of adults HBsAg + and HBeAg positive. View Table 4
Figure 3 showed that Hepatitis B virus infection was highest in the age group 26-45 and thereafter declined with increasing age (Figure 4).
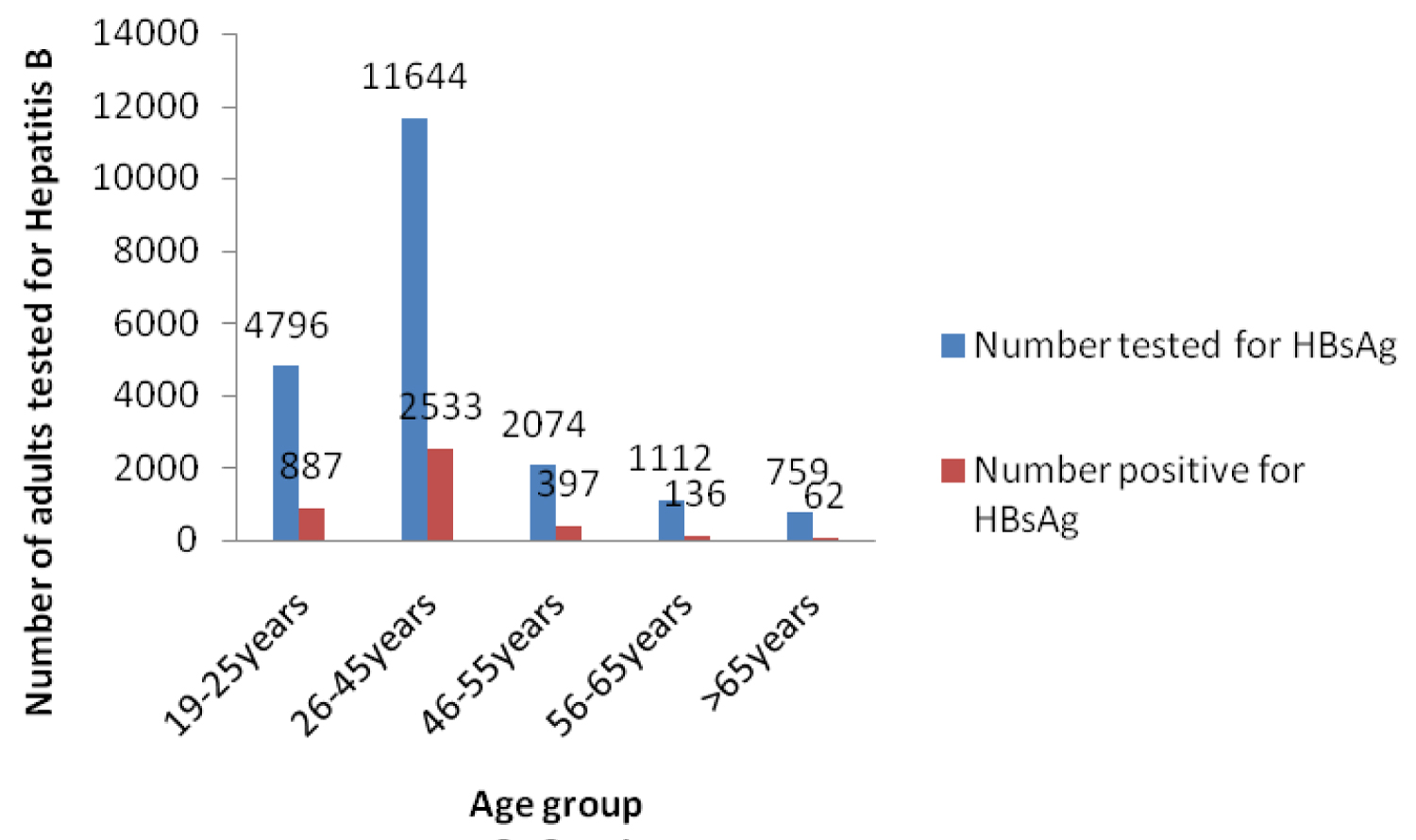 Figure 3: Adults tested for HBsAg and Positive for HBsAg 2000-2015.
View Figure 3
Figure 3: Adults tested for HBsAg and Positive for HBsAg 2000-2015.
View Figure 3
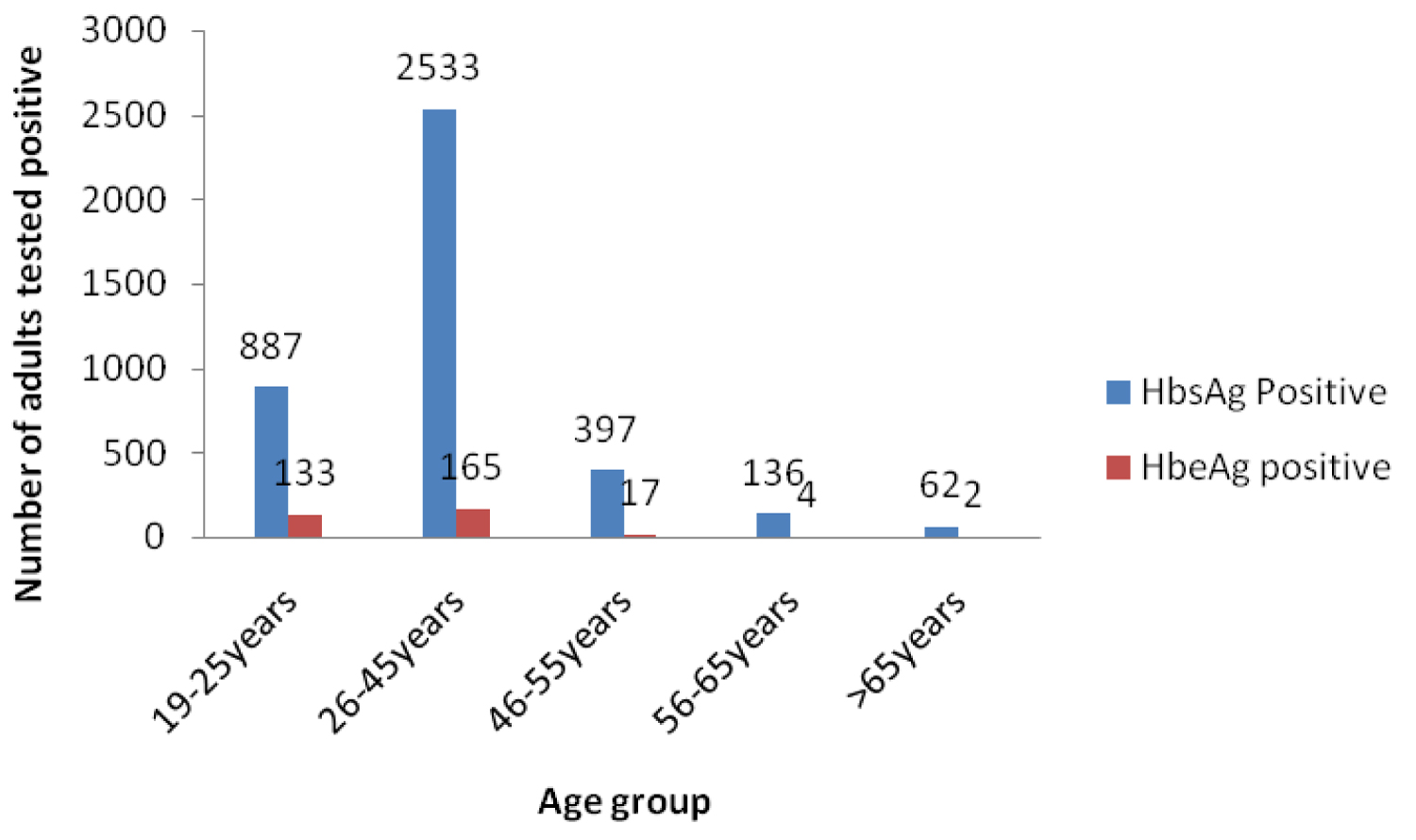 Figure 4: HBsAg and HBeAg positive subjects during the period 2000-2015.
View Figure 4
Figure 4: HBsAg and HBeAg positive subjects during the period 2000-2015.
View Figure 4
To the best of our knowledge this report represents the highest number of adults tested for Hepatitis B and HB e antigen in Nigeria from a health facility. The prevalence of HBsAg in this study was 19.7%. This is higher than the pooled prevalence of 14% in various adult subgroups in Nigeria between 2000 to 2013 by Musa, et al. [10] and also higher than the 12.2% [29] prevalence in the National survey of Hepatitis B in the general population in the country in 2016.
The prevalence of 19.7% in our study is higher than recent Hospital reports of 11.4% from Kano [30] and 14% from Kaduna [12] but comparable to 19.2% from Jalingo [11] however lower than reports of 25% in Kogi [14], 39% in Makurdi [15] and 63% from Sokoto [13]. The North East Sub-region, where our study centre is located has the lowest routine vaccine coverage and highest levels of poverty, amongst other poor health indices, in Nigeria [7,8].
Nigeria like the rest of sub Saharan African countries is hyperendemic for Hepatitis B where prevalence is greater than 8% in the general population [1,2,10,29].
Ligani, et al. [31] in a systemic review and meta-analysis of HBsAg infection in Burkinasso between 1996 and 2017 found an overall and a pooled prevalence of 11.2% and 9.4% in the general population respectively. A pooled prevalence of Hepatitis B Virus of 12.3% in general population was reported in Ghana [32] between 1995 and 2015. This high HBsAg prevalence was reported in similar systematic review and Meta-analysis in Cameron [33] and Malawi [34] between 1996- 2018 with prevalences of 11.2% and 8.1% respectively. While these reviews included hospital based data that used different testing methods and were not sex disaggregated, HBsAg infection presents enormous health challenges with profound implications for social and economic wellbeing of Africa countries [1,2,35].
In our study HBsAg infection prevalence was higher in males compared to females and this was statistically significant. This finding is similar to earlier and recent reports from Nigeria [10-15,29] and indeed to studies from Burkina Fasso [31], Ghana [32], Cameron [33] Malawi [34].
Plasma clearance rate for HBsAg in males is slower compared to females and females were said to have elaborated more antibodies to HBsAg than males [36]. Differences in tribal and sexual behaviours between males and females may account for higher percentage of HBsAg positive males with HBV chronic infection [37]. An Altered Pattern of Liver Apolipoprotein A-I Isoforms is implicated in male chronic Hepatitis B progression [38].
Age at infection is known to influence the establishment of HBV infection [39]. In Asia Infection persists in 80-90%, 20-30%, 10%, and 5% of people infected perinatally, in early childhood, adolescence, and adulthood, respectively [40]. Compared to Asia, the risk of mother to child transmission of HBV in Africa is low with a pooled risk of 38.3% among HBeAg positive pregnant women [41].
In our study, while majority with HBsAg were young and middle aged adults, determining infection trajectories would be difficult as there is no routine screening and intervention for Hepatitis b pregnant women; Hepatitis B vaccine coverage in Nigeria, introduced in 2004, is abysmally very low [7,8] and horizontal transmission of HBV during childhood in Sub Saharan Africa is significant. Early HBV infection may increase the risk of liver cirrhosis and/or HCC through the persistence of high viral replication, in addition to increasing the risk of chronic infection [39,42,43].
A significantly high prevalence of hepatitis B in proportion of young and middle aged adults in our study may be associated with perinatal transmission [1,2], lack of HBV immunization [7,8] or early childhood and adolescent transmission [39,40]. While some of our subjects were recruited before the start of HBV vaccine in Nigeria in 2004, common risk factors for hepatitis B infection in Nigeria were uvulectomy, presence of tribal marks, sharing of sharp objects, and circumcision, which is performed traditionally on some Nigerian males [29].
Sero-conversion of HBsAg is slow and in West Africa Shimakawa, et al. [44] reported an HBsAg clearance of 1.0%/year with half clearing by 57 years old. Younger age and high HBV DNA levels at baseline were associated with delayed HBsAg sero-conversion [44].
Before the availability of The HBV Combo Rapid Test Cassette Kit for the rapid diagnosis of HBsAg and other markers of hepatitis B (HBeAg; AbHBe, AbHBs and AbHBc), kits for hepatitis b and envelope antigen were separate in our environment. It's probable a combination of factors of cost of envelope antigen test, low awareness on the significance of the HBeAg test and or inadequate patient counseling accounted [1,2] for why only a third of HBV carriers tested for this marker of viral replication, infectivity, inflammation, severity of disease and response to antiviral therapy.
The prevalence of HBeAg among chronic HBsAg carriers in our study is higher than earlier reports of 16.4 % by Amazigo in Eastern Nigeria [17]; 8.8% by Abiodun, et al. in BeninCity [18]; 10.8% in Ibadan by Otegbayo, et al. [19]; Lesi, et al. in Lagos 11.9% [20]; 19% and 8.6% by Ola, et al. [45] and Ijoma, et al. in Enugu [21] respectively but much lower than other earlier reports of 48.4% by Ojo, et al. [46] in patients with liver disease and Mbaawuaga, et al. [47] of 30.3% amongst pregnant women in Makurdi.
Later reports of HBeAg from Lagos by Akinbami, et al. [48] Forbi, et al. from Central Nigeria [22], Odimayo [23] and Mbaawuaga, et al. [49] both from Makurdi of 8.2%, 19.2%, 3% and 9.3% respectively were lower than our study findings. Iroezindu, et al. [50] from Jos reported comparable prevalence of 28% in HIV co infected patients but lower HBeAg of 15% in control subjects. Yakasai [51] from Kano however found HBeAg seroprevalence of 62.5% among pregnant women who tested positive for HBsAg and 26.01% among non-pregnant women attending the gynecology clinic in the tertiary health facility. However, in recent studies Abah, et al. [52] reported HBe prevalence of 6.5% amongst pregnant women in Kano; Francisca, et al. [53] from Nnewi and Okeke, et al. [54] from Zaria reported HBeAg prevalences of 30.7% and 11% respectively. While most of these study findings have lower HBeAg figures than our study, in general they were very much limited in sample sizes, age disaggregation, age range and period of study but no doubt significant contributions to understanding this infection.
Hepatitis Be antigen was reported as 13.3% in Ghana [55] and 12.8% in India [56] amongst Chronic Hepatitis B surface antigen carriers. Testing methods were different and subpopulations varied. Merrill, et al. [57] in a comprehensive review of the prevalence of HBV seromarkers in subpopulation groups across the 14 regions of WHO demonstrated that HBsAg and HBeAg testing methods affected prevalence. This observation was also reported by Musa, et al. [10] in national review of Hepatitis in the country. Merrill, et al. [57] reported a wide variation in Hepatitis Be antigen prevalence amongst WHO sub-regions.
In our study, HBeAg was predominantly in young and middle age adults and decreased with increasing age with more males than females affected. Clinically, HBeAg is an index of viral replication, infectivity, inflammation, severity of disease and response to antiviral therapy [6].
During the natural course of chronic hepatitis B virus (HBV) infection, patients with high serum levels of viral DNA and hepatitis B e antigen (HBeAg) may gradually and spontaneously clear HBeAg and develop antibody to HBeAg [58]. In contrast to HBeAg loss, clearance of HBsAg rarely occurs in patients with chronic hepatitis B, the annual rate of HBsAg clearance in adults was reported to be 0.5%-0.8% [59]. Persistence of high HBV viral load or envelope antigen play an important role in increasing the risk of primary liver cancer [60,61].
Cheng, et al. [60] reported that patients with HBeAg seroconversion before the age of 30 years have excellent prognosis, whereas patients with delayed HBeAg seroconversion after age 40 have significantly higher incidences of HBeAg-negative hepatitis, cirrhosis, and Hepatocellular carcinoma.
In West Africa, Shimakawa, et al. [44] reported age-specific prevalence of HBeAg at baseline decreased with increasing age; of the 173 HBeAg-positive carriers at baseline, 82.1% lost HBeAg and the clearance rate was 66.4%/year. Maternal BeAg and genotype C were associated with delayed HBeAg seroconversion [62].
Even though, only a third of our subjects tested for HBe antigen, thereby underestimating its burden, the significance of e antigen in the treatment decision in sub Saharan Africa has recently been suggested [16].
The prevalence of both HBsAg and HBV e antigen in adults in our report is high and in particular the prevalence of HBV e antigen in our study population may have been under estimated. Clinical and economic implications are profound as its epidemiology is crucial in HBV transmission and prioritizing access to treatment especially in Sub Saharan Africa.
The limitations of our study are several: As this was a one point screening test we could not determine if the cases detected were those with chronic infection (HBsAg for > 6 months). We could not report liver function and liver status including Biopsy and Hepatitis B viral load tests results. Neither could we report Anti-HBs or Anti-HBc test status which could have defined unexposed adults requiring Hepatitis B vaccine. Viral load test started in 2019 in our centre and currently beyond the reach of most. A nationally approved treatment guideline has not yet been lunched.
A prospective nationally representative multicenter longitudinal study on Viral Hepatitis Epidemiology and treatment is urgently required in Nigeria. Legislation making 3-dose Hepatitis B vaccine including Routine immunization mandatory is needed in Nigeria and indeed some sub-Saharan countries.
We wish to acknowledge Hajiya Fatima and Hajiya Zainab Danmalam of the data unit of paediatrics department for extracting the data.
The authors declare no conflict of interest.
Isaac Warnow Elon: Conceived of the study and study design, developed the first manuscript draft, and critically reviewed all drafts of the manuscript.
Ajani Ayomikun: Oversaw the study design, conducted quantitative data analysis, developed the first manuscript draft, and critically reviewed all drafts of the manuscript.
JaloIliya, Alkali Yaya: Oversaw the study design and critically reviewed and commented on the final manuscript.
Abubakar Joshua Difa, Oyeniyi Christianah: Conducted quantitative analysis and commented on all drafts of the manuscript.
Aremu John, Danlami Halilu: Critically reviewed and commented on the final manuscript.
The ethical clearance for this study was obtained from the ethical committee of the Federal Teaching Hospital, Gombe.