N. meningitidis is recognized as the cause of Infection Meningococcal Disease (IMD) generally develops rapidly 20% of survivors suffer from neurological and disabling sequelae in spite of prompt antibiotic therapy. The disease is a major concern in public heath worldwide and can occur as sporadic cases, outbreaks, and large epidemics. Although in Mexico the invasive meningococcal disease is notifiable, reports about meningococcal diseases are scarce with no data available related to the presence of genotypes or "hyper invasive lineages".
To review the epidemiological characteristics of Neisseria meningitidis isolates from patients with IMD.
Biological samples were obtained from patients with a diagnosis of IMD in nine different hospitals in Metropolitan Area of the Valley of Mexico (MAVM) between 2010 and 2011. Susceptibility test to penicillin, cefotaxime and ciprofloxacin were determined and the genetic diversity of the isolates was analyzed.
Thirty-eight samples were analyzed, which were identified as N. meningitidis; ten by bacteriological culture and 14 with polymerase chain reaction (PCR), of the total of the 24 positive, 20 were serogroup C, two serogroup W and there was one of serogroups B and Y. Eight of 10 culture positive samples were identified as penicillin resistant serogroup C and epidemiological and molecular links were also found between these isolates with multilocus sequence typing grouped as Clonal Complex 11.
A low recovery rate of positive cultures was evident, we have shown that the diagnostic support of PCR in patients in who's clinical and epidemiological data were conclusive for a definitive diagnosis. The 80% of the isolated analyzed (8/10) were resistance to penicillin. Epidemiological and molecular links were found among isolates of serogroup C.
N. meningitidis, Mexico, Serogroup, Meningococcal disease, PCR, MLST, Clonal complex
Neisseria meningitidis is recognized as the cause of Infection Meningococcal Disease (IMD) generally develops rapidly 20% of survivors suffer from neurological and disabling sequelae in spite of prompt antibiotic therapy. The disease is a major concern in public heath worldwide and can occur as sporadic cases, outbreaks, and large epidemics [1]. Studies in the areas of pathogenesis, epidemiology and molecular microbiology have shown that meningococcal strains associated with epidemics and outbreaks generally belong to uniform clonal complexes (CCs), in contrast to more variable meningococcal strains that cause sporadic disease [2]. An increase in the incidence of IMD caused by Neisseria meningitidis W has been observed in several countries worldwide (South America, Europe, Australia and some parts of Saharan Africa) [3]. Since 2012, there were already reports in Chile related to the presence of change of serogroups in IMD with MenW [4].
Currently, the data on the epidemiology of meningococcal disease is limited in Mexico, which has a reported a low incidence of 0.06/100,000 inhabitants [5]. The mean and median annual cases reported through the National Epidemiological Surveillance System (Sistema Nacional de Vigilancia Epidemiológica - SINAVE, per initials in Spanish) from 1990 to 2003 were 26 and 18.5, respectively, which is likely an underestimation of the true burden of meningococcal disease in Mexico and since June 2009, the SINAVE [5] began reporting an increasing number of cases in the metropolitan area of Mexico City. However, the true burden of Meningococcal Disease (MD) is unknown. Not all isolates are submitted to the national reference laboratory. As a consequence, a limited number of isolates receive further characterization. In general, the number of reported MD cases has increased since 2002. Thus, meningococcal disease in Mexico has been considered a rare infection, reported as sporadic cases and small outbreaks, with serogroup C being the most prevalent [5].
Although there are meningococcal vaccines that allow controlling the disease, have not been included in routine programs in Mexico. Since 2010, a national response strategy has been developed that includes the availability of vaccines, but they are only used in case of outbreaks and, more recently, offered for travelers to high-risk countries.
In March 2011, Chacon, et al. report that in 27 months a total of 14 confirmed cases of invading meningococcal disease was described in the Civil hospital of Baja California, México with predominance of serogroup C [6].
Laboratory-based diagnoses of suspected cases of IMD in Latin America occur almost exclusively via culture without antibiogram, in the absence of standardized strategy based on nucleic acid amplification techniques and meningococcal genotype studies [7].
The purpose of the study was to describe the epidemiological characteristics of N. meningitidis isolates from patients with invasive disease in nine hospital of the Metropolitan Area of the Valley of Mexico (MAVM) between 2010 and 2011, after the unusual increase in cases of meningococcal disease identified in the country in 2009.
All patients suspected of invasive meningococcal disease (IMD), characterized by symptoms related with meningitis (sudden onset fever, headache, stiff neck, and/or nausea or, vomiting) and/or by clinical features of meningococcal (purpuric rash of the disease), were received at nine hospitals within the MAVM and recorded by the surveillance network for meningococcal disease. During a period of 23 months (January 2010 to December 2011), CSF and blood samples were collected and processed to identify N. meningitidis. A questionnaire was completed for each patient to obtain data such as age, sex and clinical picture compatible with IMD, Informed consent was also obtained from the parent(s) or guardian of each participant in accordance with the "Ethical Principles for Medical Research involving Human Subjects" of the Declaration of Helsinki [8]. The study was submitted and accepted by the ethics and research committees of each of the participating centers.
Samples were processed according to classic microbiological procedures [9] to identify N. meningitidis isolated from blood or cerebral spinal fluid (CSF). Serogroup identification for each isolate was determined using the agglutination technique [9] with group-specific antisera (Difco™). Susceptibility tests to antibiotics for therapeutic and prophylactic purposes (penicillin, cefotaxime and ciprofloxacin) were conducted according to guidelines set down by the Clinical and Laboratory Standards Institute [10].
This test was performed as described by Bennett, et al. [11], on all CSF samples, blood and bacterial cultures of N. meningitidis to confirm and/or identify serogroup DNA from N. meningitidis cultures and biological products was obtained and purified using the Wizard® DNA Purification System (Promega, Madison, WI, USA). PCR amplified products were visualized by gel electrophoresis in 2% agarose gels stained with ethidium bromide.
All bacterial genomic DNA was prepared in agarose plugs and digested with the restriction enzyme SpeI and DNA digested were separated by electrophoresis in agarose 1% w/v gels as described by Bygraves, et al. [12], to generate macro restriction fragments of chromosomal DNA, which were then processed using the CHEF-MAPPER Pulsed-Field Gel Electrophoresis System (Bio-Rad™), under the following conditions: 6 volts/cm/1-30 seconds/17 hours, followed by 6 volts/cm/1-30 sec/8 hours. Gels were stained with ethidium bromide and photographed under UV light at 320 nm using a Gel Doc image documentation system (Bio-Rad™). PFGE banding patterns were compared using the criteria of Tenover, et al. [13].
Group C isolates were typed as described by Maiden, et al. [14], and each of the isolates was assigned sequence types and corresponding clonal complex (CC) according to the Neisseria MLST internet portal (http://pubmlst.org/neisseria/).
Statistical analyses were performed using SSPS software version 12 for Windows. Results are presented as frequencies and proportions with their corresponding Phi correlation coefficient.
During the period from January 2010 to December 2011, 38 patients with suspected IMD were identified, from whom 27 CSF and 11 blood samples were obtained. None vaccinated against N. meningitidis.
Of the 38 cases, 13.1% (n = 5/38) were hospital in-patients < 12 months of age (mean ± 5.6 months, SD = 4.98). In the remaining 86.8% cases (n = 33/38), the age range was 1 to 65 years (mean 20.5 ± years, SD = 17.03). The percentage was higher in women (60%) (Table 1).
Table 1: Demographic characteristics of patients with a clinical diagnosis of Invasive Meningococcal Disease in Mexico City during 2010-2011. View Table 1
Twenty four (63.2%) samples were confirmed by PCR as positive for meningococcal infection and 14 (36.8%) others were not. Of the 38 cases, 10 samples showed positive results by culture and were also confirmed by PCR. Eight isolates were identified as serogroup C and showed 100% susceptibility to cefotaxime and ciprofloxacin and 100% resistance to penicillin at 1.5 μg/mL, which correspond the 80% of the isolated analyzed (8/10). Of the two remaining cultures, one was positive for serogroup B and the other for serogroup Y. The other 14 samples were positive only by PCR, giving a final number of 20 samples in group C, 2 in W (Table 2).
Table 2: Number of patients with invasive meningococcal disease by hospital, serogroup and diagnostic technique for N. meningitidis cases reported in the Metropolitan Area of the Valley of Mexico between 2010 and 2011. View Table 2
The Phi correlation coefficient was used to determine the correlation of variables. It was found that the proportion of cases identified by PCR was different from the cases identified by culture (rØ = 487, p = 0.003).
The genetic interrelationship of the subgroup C strains of N. meningitidis was examined by PFGE. Three different patterns were observed, all of which were closely related (Figure 1).
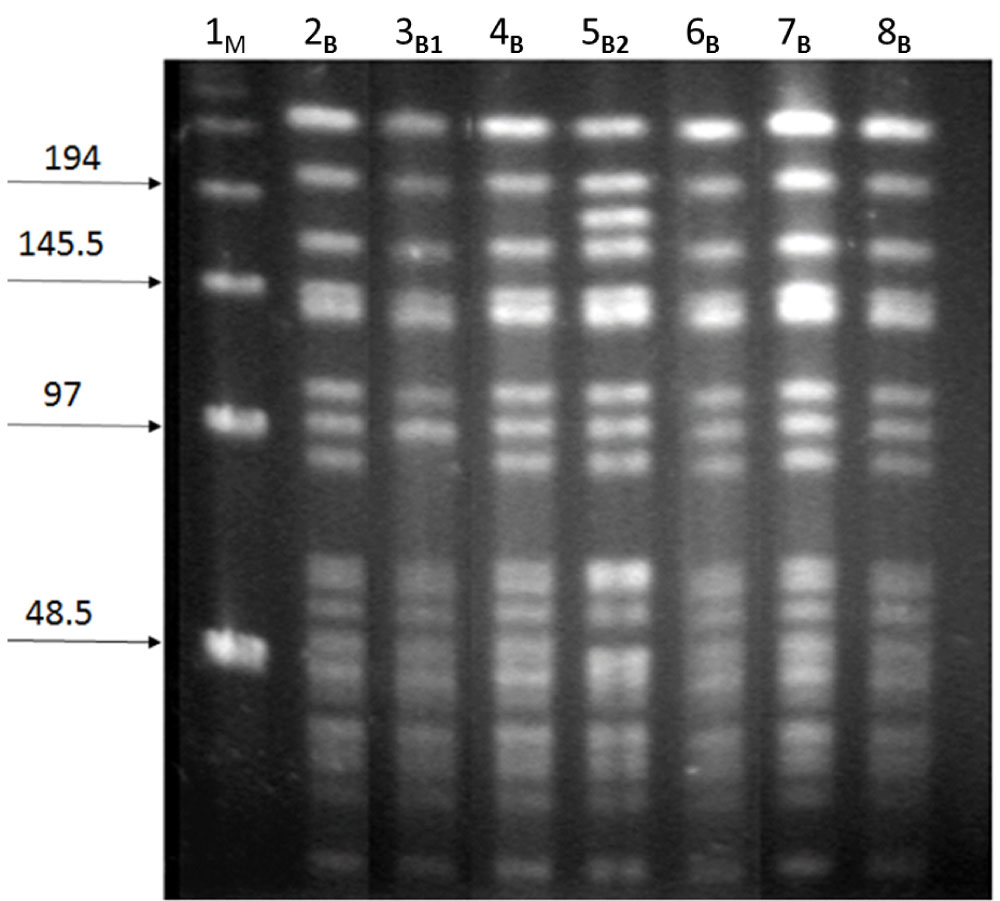 Figure 1: Electrophoretic patterns of N. meningitidis serogroup C isolates generated by PFGE (B, B1, B2). M: Lambda ladder molecular weight marker; lanes 2 to 6: Strains of N. meningitidis group C isolated from patients with meningococcal disease during the study period (2010-2011).
View Figure 1
Figure 1: Electrophoretic patterns of N. meningitidis serogroup C isolates generated by PFGE (B, B1, B2). M: Lambda ladder molecular weight marker; lanes 2 to 6: Strains of N. meningitidis group C isolated from patients with meningococcal disease during the study period (2010-2011).
View Figure 1
Using the Neisseria database PubMLST, the sequence types (STs) of each of the isolated strains of serogroup C were assigned to the CC 11.
Invasive meningococcal disease can be very serious; the clinical scenarios are identified as meningococcemia, meningitis or meningococcemia with meningitis. Meningococcal disease has a rapid onset with potentially life-changing consequences. IMD is fatal in as many as 50-80% of untreated cases, and case fatality rates even in treated individuals are 10-15%. In addition, IMD causes great morbility, with 12-20% of survivors suffering significant clinical sequelae, mental impairment, amputations, and seizures [15].
The IMD is a disease preventable by vaccination and at present, there are different immunization programs with meningococcal vaccines, however, in Mexico, this vaccine is not included in the basic vaccination schedule in the country and can only be acquired through private medicine.
In Mexico, the regulations are such that cases of meningococcal meningitis are a cause for immediate mandatory reporting, but not so for cases of meningococcemia [5].
In a study conducted by Chacon, et al. [16] in Tijuana, Baja California, Mexico, the highest infection rate (47%) in cases identified between 2005 and 2008 occurred in the 1-4 year age group with 62% attributable to serogroup C.
Similarly, in the present study, we identified that 43% (9/25) of infections were in the same age group.
In a carriage study conducted in Mexico in 2005, Espinosa, et al. [17] reported that 1.6% of carriers of N. meningitidis were part of various closed populations, such as children under five years and teenagers (identifying serogroups Y (29.7%), C (24.3%) and B (10.8%) as well as the presence of the CC 11 before ET-37 complex of N. meningitidis.
According to reports in the literature there is a difference of the circulating serotypes but similar clonal complex (CC11) in Mexico, with those of other countries such as Europe, where the total incidence of invasive meningococcal disease (IMD) was considered to have been decreasing in recent years; however, a rising incidence of IMD caused by serogroup W (MenW) was reported in the United Kingdom (UK) in 2009, then the Netherlands and Sweden, following the spread of MenW IMD in South America since 2004. The isolated strains predominantly belong to the multilocus sequence typing (MLST) defined hyper virulent clonal complex clonal complex 11 (CC 11) [18].
The epidemiology of IMD is dynamic, eith continuing changes in incidences of N. meningitidis serogroups and the emergence of new strain variants, uses a variety of mechanisms to undergo antigenic variability, particularly in the face of natural or vaccine induced immunity. This antigenic variability occurs mainly through horizontal gene transfer, wich allows the organism to acquiere large DNA sequences. The meningococcus also uses gene conversion, wich is autologous recombination and does not requiere the acquisition of DNA from another strains. N. meningitidis is also capable of variaying its antigenic profile through variable gene expression and fase variation. Capsular switching is the mechanism by wich N. meningitidis can change its capsular phenotype. Meningococcal outbreaks can be started or sustainbed by capsular swiyching, wich is believed to allow immunologic escape from the original serogroup. Capsular switching occurs through horizontal gene transfer and is detected by identyfying strains that are related geneticacally as defined by for example MLST but express differenty capsular polisaharide [19].
This is the importance of complementing the phenotypic study with the molecular analysis of N. meningitidis isolates.
The low frequency of reporting of meningococcal disease and the difficulties inherent in the diagnosis generated by the low sensitivity of the methods commonly available may be important factors in the underreporting of the disease in Mexico. Another reason that could explain the low reported frequency is the immunity of the population to the strains currently circulating in the country, changes in the prevalence of risk factors, behavioral factors and unknown variables, as has previously been reported [20,21].
We have shown that the diagnostic support of PCR in patients whose clinical and epidemiological data were consistent was conclusive for a definitive diagnosis, as only 26.3% of the cases included in this study met the criteria of the Health System of Mexico necessary to be considered as confirmed cases of IMD; with PCR applied as a diagnostic tool, the percentage of cases confirmed increases to 63.5%.
It should be noted that Mexico's health system defines a confirmed case of meningococcal disease only when there is a positive culture, which is likely a cause of the low incidence reported. However, there is a theory to explain, why the low incidence of N. meningitidis in some countries such as Mexico and is likely to arise as a result of enteric exposure to other bacteria that express cross-reaction antigens [22].
Thus, we consider it necessary to review these criteria to permit the inclusion of a confirmatory diagnosis by incorporating PCR into diagnostic criteria as already occurs in other countries such as Brazil [23] and the United Kingdom [24], where a significant number of cases are diagnosed without culture; this has improved detection of meningococcal disease generally.
Hence, the GMI recommends that confirmatory PCR be instituted wherever possible, but without replacing culture or other methods. In recent years,new PCR and DNA sequencing methodologies have enhanced the characterization of N. meningitidis strains. DNA sequencing methodologies have been used for molecular determination of sensitivity to antimicrobials through identification of allelic variants of specific genes and sensitivy/resistance to the corresponding antibiotics [25].
Specific antigen sequence typing (AST) to characterize the variable regions of N. meningitidis, sucha as the antigen encoding genes por A and fet A. As whole-genome sequencing technology becomes more widely available it is likely to supersedes MLST and AST, enabling even more detailed strain characterization [25].
Genetic analysis of serogroup C strains showed a very similar electrophoretic profile investigated in the present study and provides evidence of the presence of a single clonal complex identified as CC 11, considered to be hypervirulent and to have caused meningitis and septicemia epidemics in other regions [18,26].
A match between the presence of CC 11 in our study and that of Espinosa, et al. [17] in 2005 was identified. From this, it is reasonable to infer that the circulation of this clone had been demonstrated in our population and assume that cases of meningococcal disease have been underestimated due to misdiagnosis or not being recognized by doctors.
This and other reports like those of Mac Neil, et al. [27], in relation to N. meningitidis CC 11, in South America, United Kingdom sub linage comprises W: CC 11 that emerged in South America and subsequently spread to the United Kingdom and Europe.
We therefore consider it important to highlight the findings of this serogroup among our population.
By investigating whether there was an epidemiological coincidence between the cases studied, we found that all patients with N. meningitidis serogroup C in our study had a close relationship with an inmate serving jail time, and although there was no analysis of this as a risk factor because it was not included in our variables, it is a compelling finding because healthy N. meningitidis carriers of serogroup C CC 11 were subsequently identified among inmates of two correctional centers in Mexico City [28].
Until this study, there were no reports in the literature of N. meningitidis strains in Mexico that were resistant to penicillin. In this report, the 80% of the isolated analyzed (8/10) were resistance to penicillin, all serogroup C isolates, were defined as resistant if they had a minimum inhibitory concentration (MIC) of > 0.06 to 0.5 mg/mL, whereas the cutoff value established by the Clinical and Laboratory Standards Institute (CLSI) [9] to consider an isolate sensitive is < 0.06 μg/mL. Other countries such as Australia [29] (84.6%) and Italy [30] (73%) have also identified resistant strains to penicillin, as we found in our study.
This study provides valuable insight into the meningococcal disease in Mexico, and demonstrates the importance of implementing more sensitive diagnostic techniques that identifies the causal agent in a timely manner, such as PCR and specific antigen sequence typing (AST) to characterize the variable regions of N. meningitidis. However the culture remains the gold standard in confirmation and characterization, as it maintains the isolate for future characterization.
Surveillance for meningococcal disease is essential to monitor and evaluate changes in disese incidence, to understand the burden of meningococcal disease, therefore, the results should be the basis of surveillance of health agencies, seeking diagnostic and preventive alternatives such as vaccination.
Surveillance network for meningococcal disease in Metropolitan Area of the Valley of Mexico (Red de vigilancia de la enfermedad meningocócica en el Área Metropolitana del Valle de México): Raymundo Rodríguez Badillo: Manuel Gea González Hospital. Sarvelio Espinosa Moreno: Federico Gómez Children's Hospital of México. Marte Hernández Porras: National Institute of Pediatrics, Norma Angélica Matías: La Raza Medical Center. Claudia López: Spanish Hospital. Ma. Elena Garcia Meza, "La Perla" Hospital, Cd. Nezahualcoyotl. Edo. Mexico. Abad Raquel, Veronica Medina. National Reference Laboratory for Meningococci, Carlos III Institute of Health, Majadahonda, Madrid, Spain.
This study was conducted with support from Sanofi-Pasteur.
Julio A. Vazquez Moreno has received research funding from Sanofi-Pasteur, Pfizer, Baxter, Novartis and GSK.
Luz Elena Espinosa de los Monteros, has received research funding from Sanofi-Pasteur.
Obtained.
The study was submitted and accepted by the Ethics and Research Committees of General Hospital and Dr. Manuel Gea Gonzalez" under study number 12-90-209.