Meningococcal disease in Mexico has a very low incidence; it is one of the countries with few reported cases as prevalence of the carrier state is unknown. Some studies have shown that many invasive meningococcal diseases are restricted to a specific number of hyper-virulent strains that contrast with carrier isolates. The aim of this study was to determine the frequency and characterization of meningococcal carriage isolates among prisoner's inmates. Nasopharyngeal swabs were obtained, Neisseria meningitidis was identified by standard methods, Polymerase Chain Reaction, and penicillin susceptibility was carried out. Multilocus Sequence Typing was performed as well. The study included 461 inmates. The overall carrier frequency was 10.41%, serogroups were isolated: B (20), C (17), Y (8) and nongroupable (3), 70.8% (34/48) of isolates showed some level of resistance to penicillin. Genetic diversity among isolates is observed and the presence of hypervirulent clones was demonstrated as ST42/44 CC and ST11/ET37CC.
Neisseria meningitidis, Pharyngeal carriage, Serogroup, Genotype, MLST, Mexico
Although N. meningitidis has the potential to cause invasive disease, it is a pathogen that colonizes human nasopharynx asymptomatically [1] and is transmitted from person to person by aerosol droplets [2].
For not well understood reasons, N. meningitidis is occasionally responsible for septicemia and meningitis [3], especially in children and young adults [4,5].
Meningococcal disease usually occurs 1-14 days after pathogen acquisition, which may also result in upper respiratory, and pharyngeal meningococcal carriage, its duration may vary from days to months [6].
Most carriage meningococcal isolates lack a capsule and are thus nongroupable (NG). However, these strains may play an important role as a reservoir of virulence genes, with implications for meningococcal diversity due to the high frequency of recombination [7].
Some studies have shown that many invasive meningococcal diseases are restricted to specific number of hyper-virulent strains that contrast with carrier isolates. This indicates that some meningococci are genetically adapted to cause invasive disease [8].
Globally, adult carrier meningococcal infection rates have been reported to range from 10-35%. It is assumed that the carrier state is quite common, although this rate varies with age, environment, and other social conditions [8].
Molecular characterization of the organisms colonizing the throat is essential to our understanding of the dynamics of transmission of N. meningitidis in the human population and, thus, the epidemiology of the disease.Some molecular methods for typing N. meningitidis have been developed and exploited in epidemiologic studies like multilocus sequence typing (MLST) to research population biology and evolution of microorganisms, PubMLST database to compare global meningococcal strains [9] and Pulsed-Field Gel Electrophoresis (PFGE) used in outbreak investigations [10].
In Mexico, meningococcal disease (MD) is reportable through the Mexican National Epidemiologic Surveillance System [11]; however, the true burden of MD is unknown. Not all isolates are submitted to the national reference laboratory. As a consequence, a limited number of isolates receive further characterization. In general, the number of reported MD cases has increased since 2002, although incidence rates are still extremely low, ranging from 0.01 to 0.04 per 100,000 in the 2010-2014 period (data from the Secretariat of Health, Mexico). Since 2010, a national response strategy has been developed that includes the availability of vaccines, but they are only used in case of outbreaks and, more recently, offered for travelers to high-risk countries. In March 2011, Chacon, et al. report that in 27 months a total of 14 confirmed cases of invading meningococcal disease was described in the Civil hospital of Baja California with predominance of serogroup C [12].
Characterization of meningococci isolated from the pharynx is essential to understand dynamics of meningococcal carriage and disease. Identification and detection of highly virulent serotypes could estimate the magnitude of the problem in our country thus, the origin of a potential epidemic outbreak could be anticipate, determining the potential impact of disease control programs such as vaccination.
The aim of this study was to determine the frequency and characterization of meningococcal carriage isolates among prisoner's inmates in two from the five prisons that exist in Mexico City.
In accordance with the provisions of the "Ethical Principles for Medical Research Involving Human Subjects" of the Declaration of Helsinki, the study was submitted and accepted by the Ethics and Research Committees.
A cross-sectional study was conducted between October and November 2010, among inmates of two of the city's main male prisons ("North" and "East"), from the five that exist in Mexico City. According to Subsecretaria de Sistema Penitenciario of Mexico City, estimated population included 26,000 prisoners in June 2010.
Necessary sample size was determined in 384 individuals for an expected prevalence of 10% with a 95%confidence level and ± 3% accuracy. To calculate the sample size, the statistical package PASS was used. Sample size in the two prisons were distributed in proportion to the prisons existing populations, and finally, 461 individuals were selected by simple random sampling.
We used of American Type Culture Collection (ATCC), for all procedures. Neisseria meningitidis: ATCC 53417: Group A: ATCC 13098, Group B: ATCC 13102, Group C: ATCC 53419, Group D: ATCC 35559, Group W135: ATCC 35560, Group X: ATCC 3561, Group Y: ATCC 35561, Group Z: ATCC 35562 and Neisseria lactamica ATCC 23970.
Flexible Dacron swabsMR (Pur-Wraps) were used to collect 461 nasopharyngeal swab samples. The samples were placed in Amies Transport Medium (BD BBL culture SwabTM plus) and immediately sent to the Microbiological Research Laboratory, where they were processed within a minimum of 3 hours after collection.
The swabs were inoculated on Thayer-Martin selective agar (Dickinson Microbiology Systems, Maryland, MD) and incubated at 37 ℃ for 24 hours in an atmosphere of 5% CO2. The plates were inspected, and 5 colonies with characteristics of Neisseria sp. were sub-cultured on blood agar medium for species identification by Gram staining, oxidase reaction, and carbohydrate utilization tests [13]. API-NH1 strips (bioMérieux, Hazelwood, MO, USA) confirmed results. The capsular polysaccharide of each isolate was determined by agglutination with group specific Neisseria meningitidis antisera W135, A, B, C, Y, Z. (BD DIFCO) [14].
Susceptibility to penicillin, cefotaxime, and ciprofloxacin (AMRESCOR) was tested by the microdilution method (prepared in the laboratory) according to the protocol established by the Clinical and Laboratory Standards Institute [15], for each isolation identified as Neisseria meningitidis.
Bacterial genomic Deoxyribonucleic Acid (DNA) was directly extracted from the N. meningitidis strains and was purified from bacterial suspensions using the Wizard® DNA Purification System (Promega, Madison, WI, USA) according to the manufacturer's protocol. The DNA was diluted in 50 µL of sterile water and multiplex PCR was performed in these as described by Bennett, et al. [16] this Neisseria meningitidis multiplex PCR assays were used consecutively that allow determination of capsular status of serogroup A, B, C, 29E, W135, X, Y and Z, in isolates by direct analysis of the amplicon size. These assays offer a rapid and simple method of serogrouping N. meningitides. PCR-amplified products were visualized by gel electrophoresis in 2% agarose and stained with ethidium bromide.
Pulsed-Field Gel Electrophoresis (PFGE). All N. meningitidis cultures (48) were processed according to the method described by J Bygavres, et al. [17] using 20 U of SpeI to generate macro restriction fragments of chromosomal DNA, which were processed with the CHEF MAPPER (Bio-Rad™) pulsed-field gel electrophoresis system under the following conditions: 6 volts/cm/1-30 seconds/17 hours followed by 6 volts/cm/1-30 seconds/8 hours. Gels were stained with ethidium bromide and were photographed under UV light at 302 nm using a Gel Doc image analyzer (Bio-Rad™). Restriction profiles were analyzed according to Tenover, et al. [18].
MLST has been developed for epidemiological studies of meningococcal by Maiden, et al. [19], to identify the alleles of seven meningococcal housekeeping genes, caused by point mutation thus recognizing genotype related groups known as Clonal Complexes (CC). The N. meningitidis isolates (48), were typed according to Maiden, et al. [19].
Each isolate was assigned a sequence type and clonal complex according to the Neisseria MLST internet portal (http://pubmlst.org/neisseria/).
A chi-squared test evaluated whether the prevalence of positive N. meningitidis cases differed depending on prison facility. The same test was used to analyze the frequency of serogroups by prison.
For the analysis of Genetic Diversity (PFGE), Bionumerics software was used (Applied Maths, St-Martens-Latem, Belgium). Isolates showing similarity > 85% were considered to be closely related. PFGE methodology was used to evaluate the genetic or clonality relationship of bacterial isolates of the carriers.
NTSYS-pc version 2.0 software was used for statistical analysis.
A total of 461 individuals, 30-65 years old (average: 47.5 years), participated in the study. None of them had been vaccinated against N. meningitidis, 296 inmates belonged to the East prison and 165 of them to the North prison. So, 461 nasopharyngeal cultures were processed, from which, 48 N. meningitidis isolates were identified. The overall carrier frequency was therefore 10.41% (48/461); the frequency in the East prison was 9.79% (29/296), and in the North prison was 11.5% (19/165). The following serogroups were found among the 29 strains of N. meningitidis isolated in the East prison: B (12), C (12), Y (4) and nongroupable (NG) (1). An 19 strains from carriers the North prision with serogroup: B (8), C (5), Y (4), and NG (2) (Table 1).
Table 1: Frequency of Neisseria meningitidis and serogroups in isolations nasopharyngeal of carrier inmates in two prisons of Mexico City, 2010. View Table 1
Chi-square value obtained was 0.176 with a significance of 0.675, which showed that the frequency for positive N. meningitidis cases was similar in both prisons.
All N. meningitidis strains were susceptible to ciprofloxacin and cefotaxime, and over 71% (34/48), were penicillin-resistant, with minimum inhibitor concentrations up to < 32 μg/ml.
Dendrogram generated with PFGE results (Figure 1), revealed 26 different electrophoretic profiles in the 48 strains of N. meningitidis analyzed, with a similar level of 0.66.
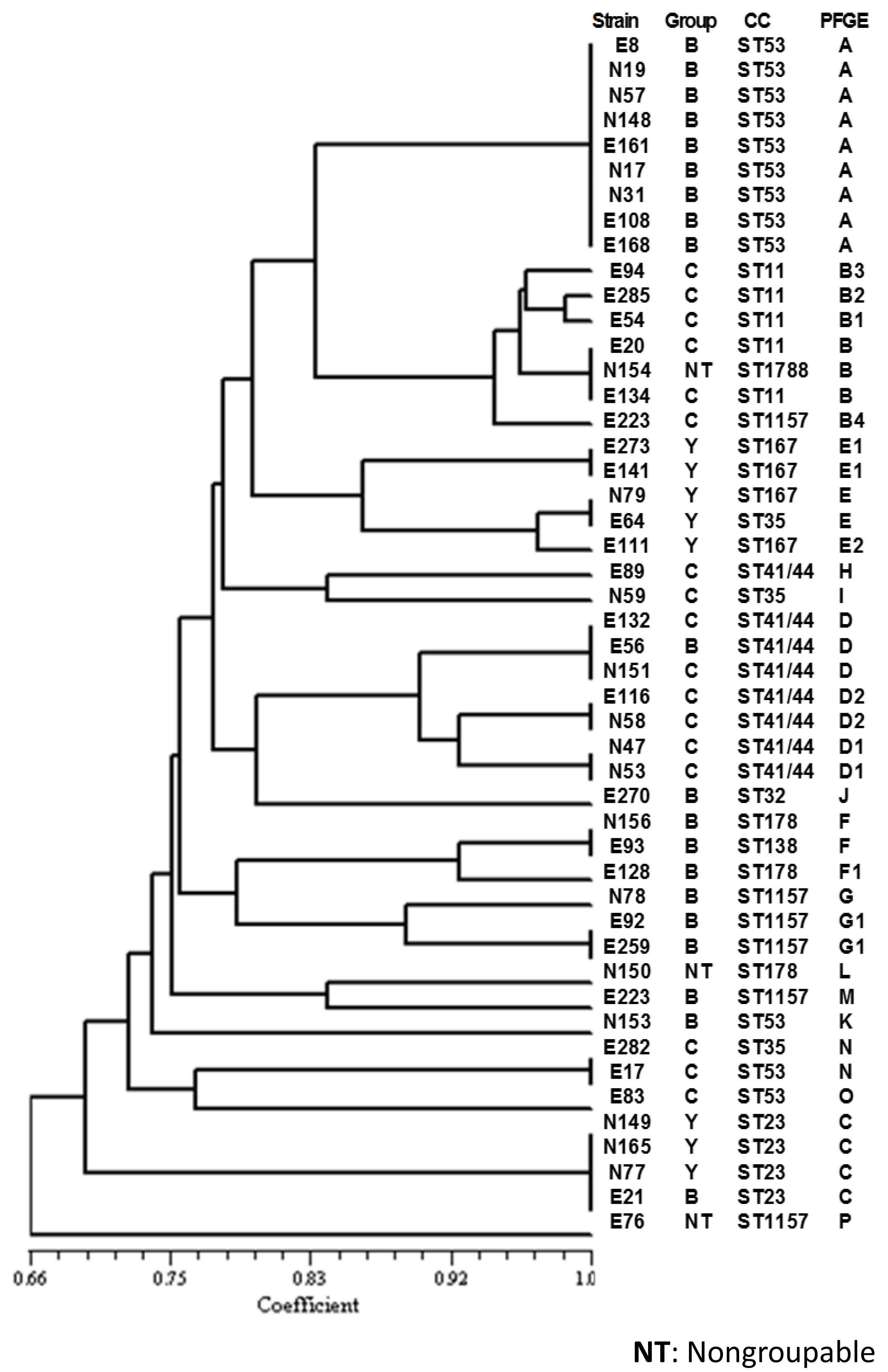 Figure 1: Dendrogram of the electrophoretic patterns generated by PFGE, of N. meningitides strains of carrier's inmates in two prisons of Mexico City, 2010. View Figure 1
Figure 1: Dendrogram of the electrophoretic patterns generated by PFGE, of N. meningitides strains of carrier's inmates in two prisons of Mexico City, 2010. View Figure 1
The most prevalent pattern, called pattern A, was found in 9 strains; 4 from East prison and 5 from North prison, all from serogroup B.
Pattern B was divided into five genetic clusters (B, B1, B2, B3, B4) based on more than 85% homology between them, as the criterion for a cluster with 6 strains, one from North prison identified as non groupable (NG or NT) and 5 strains, from the East prison from serogroup C.
The sequence types (ST) of each isolate were assigned to the available clonal complexes in the Neisseria PubMLST database and Table 2 shows the phenotypic characteristics of the clones identified.
Table 2: Phenotypic characteristics of Clonal Complexes of N. meningitidis isolated in carriers, Mexico City (2010-2011). View Table 2
Additional characterization by MLST indicated clonal complexes that reflect the epidemiology of cases of invasive meningococcal disease around the world.
Ten different STs were identified, the most frequent clonal complex was ST53, in 20.8% (10/48), as well as the existence of hypervirulent clones such as ST-41/44 CC in 16.6% (8/48) and 10.4% (5/48)ST11 CC, among others.
Meningococcal disease originates from the oropharyngeal niche through contact with a carrier. In this study we evaluate the presence of nasopharyngeal carriers of N. meningitidis of unvaccinated prisoner's at two prisons in Mexico City, we found of cultured isolates was able to provide a precise picture of both genogroup and isolate identity. Specifically, we were able to closely track isolates within the population and could assess that there was a large diversity by their genetic markers (e.g. CC).
Important to notice that most capsular groups related to invasive diseases (B, C, and Y) were found among the population studied.
The current study determined nasopharyngeal N. meningitidiscarriers' rate in 10.41% (48/461), significantly higher than reports by Espinosa, et al. [20], who mentioned 1.6% prevalence in Mexican unvaccinated children and adolescents in 2005, finding strains from serogroup Y (29.7%) as the most common ones, while our study identified predominant serogroup B (41.6%, or 20/48) and serogroup C (35.4%, or 17/48) as the most common varieties.
Although there are limited published data on carriage of N. meningitidis in Latin America, four carriage studies, a study among adolescents (n = 1208, age 11-19 years) in Campinas, Brazil, showed an overall carriage rate of 9.9%, with dominance of serogroup C (1.3%) and the highest prevalence (12%) in older adolescents (age 17-19 years). In addition, a study of healthy Chilean university students, using only culture, found an overall carriage rate of 4% [21].
Similarly, carriage of N. meningitidis was found to be 1.5% in a sample of school children in Venezuela. Understanding carriage of the meningococcus represents a major know ledge gap both regionally within Latin America and globally, with various key issues requiring further investigation that are also currently hampered by a lack of standard methodology [22].
Exist differences in epidemiologic data notification and registration protocols across Latin American countries, Brazil presented the highest Meningococcal Disease incidence rate per 100,000 inhabitants (1.9) among and Mexico reported the lowest incidence rate (0.06 cases per 100,000 inhabitants) [23].
Meningococcal Disease is a mandatory notifiable disease in all Latin American countries. However, restrictive case definitions may contribute to low reporting rates in some places. Countries such as Mexico where the notification of a case is based on a positive culture for meningococcus or an epidemiological link with a case confirmed by laboratory, this may also contribute to low reporting rates in the country [22].
In Mexico, penicillin-resistance frequency among N. meningitidis isolates is not acknowledged. Thus, it is important to note that a high incidence: 71% (34/48), was identified in this study. Penicillin-resistance was particularly high in serogroup C isolates, with 83% (15/18) incidence, compared with meningococcal disease isolates data from Argentina [24] in 2010, where penicillin susceptibility was 35.4% with intermediate resistance.
Molecular genetic studies of N. meningitidis isolates from carriers are required in order to elucidate their genetic diversity as well as worldwide distribution of this bacterium [25]. Besides, MLST isolates from carriers, showed that a large proportion of them were not CCs, previously associated with disease, like ST53 complex [26] (10/48; 20.8%).
Some studies have shown that many invasive meningococcal diseases are restricted to a specific number of hyper-virulent strains that contrast with carrier isolates that have never been associated with the disease [27]. This indicates that some meningococci are genetically adapted to cause invasive disease.
This study is in agreement with a wide genetic diversity among asymptomatic carriers, and also demonstrates hyper virulent clones as ST42/44 CC, in 16.6% (8/48),considered the cause of the disease in several countries like, USA or New Zealand, where it has been responsible for epidemics since 1991 [28].
It is assumed that isolates of N. meningitidis serogroup C, ST11 CC, present a high-level expression of the capsule, also considered hypervirulent and responsible for an invasive disease but with low frequency in asymptomatic carriers, even in outbreak/epidemic waves [26,29].
However, serogroup C ST11 CC prevalence in our study was 10.4% (5/48), this finding might reflect the type of population (closed) in which the study was conducted. We know that N. meningitidis is transmitted by direct person-to-person contact via micro drops of pflugge that contain the meningococcus, and that this increases in closed populations like our population study [27].
Up to now, these are the only reports available on meningococcal carriers in Mexico so it is difficult to estimate carriers' precise prevalence. Accordingly, any information related to N. meningitidis epidemiology in Mexico is relevant since it will contribute to elucidate transmission mechanisms.
The overall carrier frequency was 10.41%, this is the first report and analysis of Mexican meningococcal isolates by molecular methods, and it is the first application of MLST in hypervirulent clones of healthy carriers.
The existence of hypervirulent clones in asymptomatic carriers, suggests that the actual number of cases of meningococcal disease may be higher, than those reported by the epidemiological surveillance system.
Although these results do not represent the magnitude of the problem in Mexico, it is possible to prevent an outbreak by establishing adequate therapeutic measures.
We thank Dr. Ignacio Federico Villaseñor Ruiz, Health Ministry of Mexico City, Mexico and Dr. Luis Manuel Jiménez Munguia, Legal Medical Services' Director of Mexico City's prisons, for their valuable support and cooperation to successfully achieve this study.
This study was carried out with the partial support of Sanofi-Pasteur and General Hospital "Manuel Gea Gonzalez".
Julio A. Vazquez Moreno has received research funding from Sanofi-Pasteur, Pfizer, Baxter, Novartis and GSK.
Luz Elena Espinosa de los Monteros, has received research funding from Sanofi-Pasteur.
Obtained.
The study was submitted and accepted by the Ethics and Research Committees of General Hospital "Dr. Manuel Gea Gonzalez". Under study number 12-90-2009.