Hepatitis B core (anti-HBc) antibody is an effective marker for occult Hepatitis B virus (HBV) infection and is an integral part of blood donor screening in many countries. This study was aimed to evaluate the prevalence of anti-HBc antibodies among voluntary blood donors in Thailand and its significance to reduce the risk of transfusion transmitted HBV infection.
A total of 3,197 voluntary blood donor samples that were pre-screened non-reactive for HBs-Ag serology and HBV ID-NAT were tested for anti-HBc Total and IgM antibody and anti-HBs titers on ARCHITECT i6000 (Abbott Diagnostics, Illinois) between May1, 2017 and September 31, 2017 at the National Blood Centre, Thai Red Cross Society, Bangkok, Thailand.
Of the 3,197 HBsAg serology and HBV ID-NAT non-reactive voluntary blood donor samples, 659 (20.6%) were found to be reactive for anti-HBc. Of these, 118 were anti-HBs negative or < 10 IU/L where as 163 had anti-HBs titers between 10-99 IU/L and the remaining 378 had anti-HBs titers ≤ 100-500 IU/L.
Overall 20.6 percent of study donor population was found to be anti-HBc reactive of which 42.6 percent had anti-HBs < 100 IU/L titers which could lead to the increased risk of post-transfusion hepatitis depending on the HBV DNA levels in the blood products and recipient's immune status. The finding of "anti-HBc alone" reactive (118/3197;3.69%) donations percentage towards higher side highlighted the need for further investigation to ensure and implement stringent screening system to prevent occult HBV transfusion transmitted infection in the country.
Anti-HBc, Anti-HBs, Occult HBV, Thailand
Blood and blood-product safety is a global issue because transfusion therapy is of vital importance in modern medicine. However, it is also an efficient route of transmitting blood-borne pathogens. Over the last two decades, great steps forward have been made on the 'zero-risk road' in transfusion medicine. Nevertheless, the likelihood of contracting viral infections such as that of hepatitis B virus (HBV), although very low, is still present. The residual risk of HBV infection is not limited to window phase donations (estimated to be of 36 days to HBsAg conversion and of 21 days to a positive ID-NAT result [1], but also extends to those collected from donors with occult HBV infection (OBI). OBI is characterized by the presence of HBV DNA in the liver (with detectable or undetectable HBV DNA in the serum) of individuals testing HBsAg negative by currently available assays [2]. Transfusion transmitted infections (TTI), although very few, have been reported from OBI donors despite having low levels of viremia (< 100-200 IU/ml) [2,3].
Introduced in the 1980s, the hepatitis B core antibody (anti-HBc) marker was initially considered for identification of 'non-A non-B' hepatitis [4]. Subsequently, and after the introduction of a specific test for hepatitis C antibody (anti-HCV), anti-HBc was used to check for both a previous exposure to HBV and those OBI cases in which HBV DNA was only intermittently detectable [5-7]. It is well known that anti-HBc is detectable during asymptomatic infections as well as throughout life after recovery from hepatitis with or without anti-HBs production [8]. Thus, routine blood donor screening for anti-HBc was implemented in the USA [9] and in some other countries such as Japan and Germany, but it is not a mandatory practice in Thailand where assays for biological qualification of blood donations include HBsAg, anti-HCV, anti-human immunodeficiency virus (HIV) 1-2, syphilis and ID- NAT testing for HBV-DNA, HCV-RNA and HIV-RNA. In the literature, the use of anti-HBc testing is highly debated and still controversial both for its validity and the cost-benefit ratio [5,10,11]. It is also generally admitted that deferring anti-HBc reactive units would too severely affect blood supply in medium- and high-endemic areas where anti-HBc prevalence in blood donors ranges between 8 and > 50%. According to the WHO prevalence estimates in 2013, Thailand had about 4.3 million chronic hepatitis B carriers (approx. 6.4% of population), but there is no data available on the anti-HBc prevalence in Thai blood donors till now. Evaluating the usefulness of anti-HBc screening is critical, particularly in the countries like Thailand that have intermediate to high hepatitis B endemicity.
Thus, the present study aimed to determine, for the very first time, the prevalence of anti-HBc antibody among voluntary blood donors in Thailand to evaluate the significance of screening anti-HBc to reduce the risk of transfusion transmitted HBV infection in Blood banks.
The present study was undertaken at the National Blood Centre (NBC), Thai Red Cross Society (TRCS), Bangkok, Thailand from May 1, 2017 till September 31, 2017. The study protocol was approved by the TRCS Ethical Review Committee. All donors signed a donor application form authorizing storage and usage of their residual serum samples in downstream research.
The study included 3,197 samples, which consisted of 807 blood samples from NBC and 2,390 blood samples from 12 Regional Blood Centers (RBC) (200 samples from 11 sites & 190 from one site) spread across the country. Left-over residual serum samples from blood donors with non-reactive results for HBsAg, HIVAg/Ab, Anti-HCV, Syphilis, HBV-DNA, HCV-RNA and HIV-RNA from routine screening were randomly collected and de-identified and stored for batch testing. ARCHITECT HBsAg qualitative II immunoassay (with analytical sensitivity as per pack insert - 0.019 to 0.020 IU/mL) and ID-NAT with Cobas TaqScreen MPX v2 for HIV- 1/HCV/HBV with the specificity of 99.9% and 95% limit of detection being 1.2 - 1.7 IU/mL for HBV DNA (as reported by manufacture) are used for routine blood screening across all the Thai Red Cross blood centers. No demographics of the donors were collected in accordance to Ethics Committee recommendations.
Qualitative assays for HBV serology (anti-HBc Total and anti-HBc IgM) and quantitative assay for anti-HBs, were performed using commercial kits on the automated ARCHITECT i6000 analyzer (Abbott Diagnostics, Illinois) according to manufacturer's instructions on all study samples. Both anti-HBc Total and anti-HBc IgM S/CO ≥ 1 results were considered reactive. Anti-HBs levels were expressed in International Units per liter (IU/L).The assay is linear from 2 to 1,000 IU/L. Serum anti-HBs titers ≥10 IU/L was considered positive following WHO recommendations.
Data were statistically described in terms of frequencies (number of cases) and percentages when appropriate. All statistical calculations were done using Microsoft Excel 2003 (Microsoft Corporation, NY, and USA).
Results of anti-HBc and anti-HBs among the studied blood donors' population are summarized in Figure 1. Of the 3,197 HBsAg and HBV ID-NAT non-reactive voluntary blood donor's samples included in the study, 659 (20.6%) samples tested reactive for anti-HBc Total antibody. All anti-HBc Total reactive samples showed sample to cut-offs (S/CO) less than 12.0 (data not shown). Of these, 8/659 (1.1%) were reactive for anti-HBc IgM along with anti-HBc Total with one exception (Table1). The anti-HBc IgM reactive S/CO were borderline, with values between 1.2-2.7. Anti-HBs antibody positive (≥ 10 IU/L) was detected in only one-third of the study population (978/3197; 30.6%) and 44.6% (436/978) of them were anti-HBc Total non-reactive.
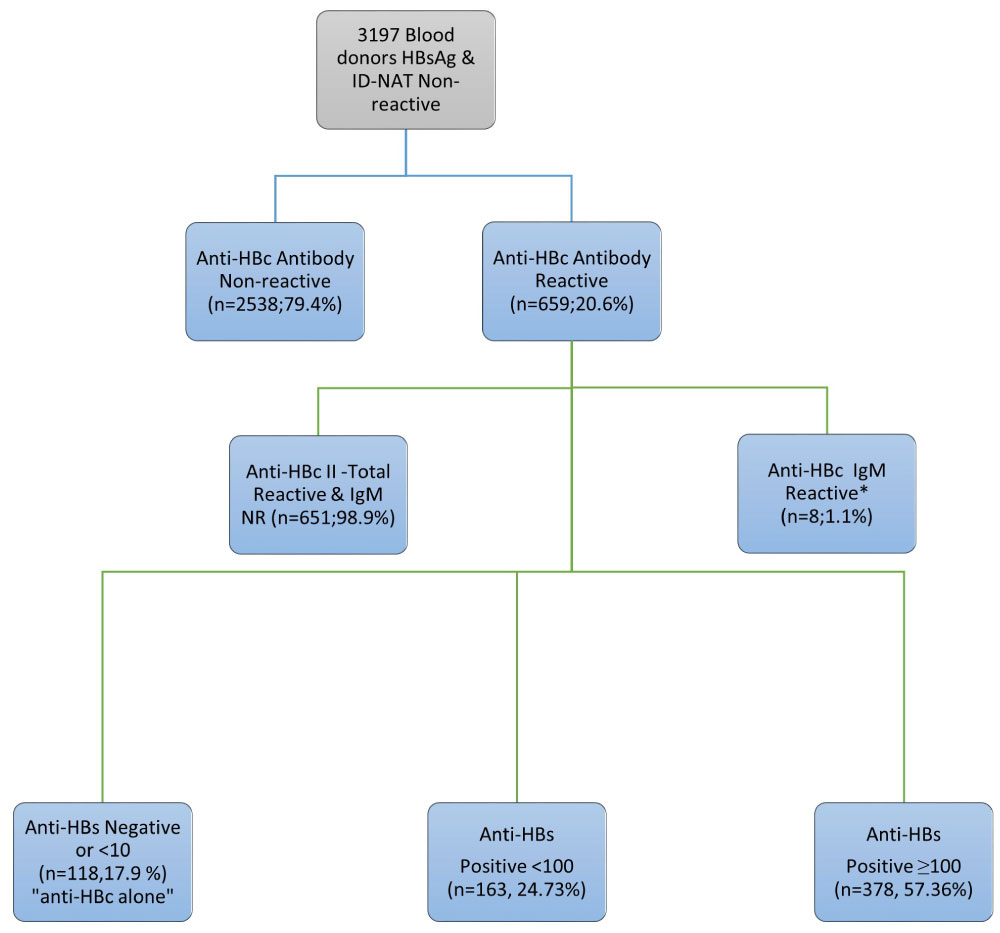 Figure 1: Schematic representation of the study population.
Figure 1: Schematic representation of the study population.
NR: Non-reactive; Anti-HBs titer in IU/l.
*7 anti-HBc IgM & Total Reactive and 1 anti-HBc IgM Reactive & Total NR.
View Figure 1
Table 1: Anti- HBc IgM Reactive Specimens Results. View Table 1
Anti-HBs antibody was detected with serum levels ≥ 10 IU/L in 541/659(82.09%) of HBsAg non-reactive and anti-HBc Total reactive samples. Figure 2 further characterized anti-HBs levels in anti-HBc reactive specimens into; negative (< 10 IU/L), low positive (≥ 10 - < 100 IU/L) and high positive (≥ 100 - > 500 IU/L) categories. There were 118 anti-HBc reactive specimens that were anti-HBs negative and 163 specimens were found to be low positive for anti-HBs levels.
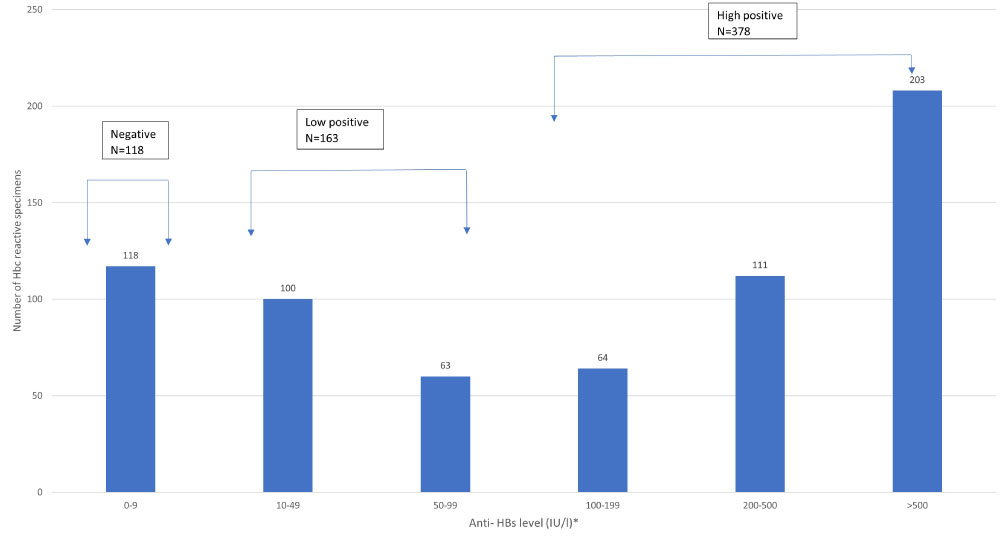 Figure 2: Anti-HBs levels in specimens tested HBsAg non-reactive, anti-HBc reactive.
Figure 2: Anti-HBs levels in specimens tested HBsAg non-reactive, anti-HBc reactive.
*No. rounded down to closest integer.
View Figure 2
Distribution of anti-HBc and anti-HBs results in the various collection sites is shown in Figure 3. The percentage of anti-HBc reactive donors ranged from being lowest (14%) in Songkhla RBC to highest (32%) in ChiengMai RBC. NBC showed 19.3% anti-HBc reactive rate being closest to the overall percentage (20.6%).
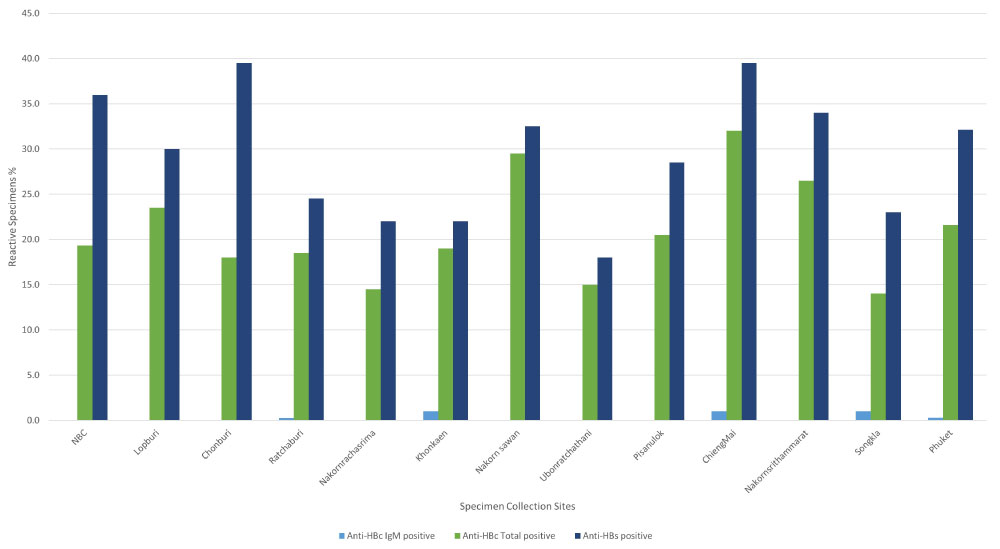 Figure 3: Distribution Percentage of anti- HBc and anti- HBs results.
Figure 3: Distribution Percentage of anti- HBc and anti- HBs results.
NBC: National Blood Centre.
View Figure 3
This study represents the first report on the screening of Thai blood donors by anti-HBc and anti-HBs antibody. We observed 20.6% (659/3197) anti-HBc reactive donations indicating high prevalence of possible past or chronic HBV infection among Thai blood donors. Consistent with reports from other high endemic countries [12,13], 57.36% (378/659) of the anti-HBc Total reactive specimens had anti-HB stiters ≥ 100 IU/L indicative of past recovered infections. Overall, the study detected 3.69% (118/3197) of samples that were anti-HBc Total reactive with anti-HBs < 10 IU/L titers (referred as "anti-HBc reactive alone"). The anti-HBc reactive alone donors could be associated with either (1) Recovering from acute infection, (2) Presence of HBsAg mutant infection, (3) Susceptible, with false positive anti-HBc testing, or (4) Occult HBV.
It is seen that acute HBV-infected subjects, in whom the anti-HBc only serological pattern is caused by clearance of HBsAg before the appearance of anti-HBs, are usually positive for anti-HBc IgM. In our study, out of 7 anti-HBc IgM and Total reactive donations, only one had low anti-HBs titer (8.02 IU/L) which could be considered in the "core window period" and the rest anti-HBc IgM and Total reactive donations had anti-HBs titer > 10 IU/L. All 8 anti-HBc IgM reactive S/CO were near borderline and hence it is difficult to rule out false reactivity. Although anti-HBc IgM is useful marker to detect recent HBV infection but its reappearance during "flares" in chronic HBV infection make it an unreliable indicator. Subjects in whom a negative HBsAg test is the result of a mutant HBV are usually viremic [14,15] while all subjects in our study were HBV-DNA negative when using highly sensitive ID-NAT assays. The possibility of false-reactivity especially among anti-HBc reactive alone low S/CO donations can't be excluded. We observed 10/118anti-HBc reactive alone with borderline S/CO (1.1- 1.8) (data not shown) which warrants secondary testing with an alternative EIA to distinguish between true- and false-positivity and to confirm borderline reactive results that might be associated with low avidity or low titer of antibodies [16]. The study didn't perform any secondary testing to rule-out false reactivity for anti-HBc and is recognized as the limitation of the study that needs to be addressed in future investigations.
HBV DNA is the only reliable diagnostic marker of OBI. Since liver specimens are usually available only in a minority of cases, analysis of serum samples is the most common approach to identify cases of OBI. In the recent report by Candotti D, et al. [17], the estimated minimum HBV infectious dose by transfusion was proposed to be revised from approximately 100 to 16 copies (or 3IU) of HBV DNA. In order to detect such low level of viral load, screening NAT should reach 0.8 copies or 0.15 IU/mL limit of sensitivity [17]. HBV ID NAT used to screen all the donations included in the study had LOD of 1.2-1.7 IU/mL which does not rule out the possibility of presence of very low or undetectable HBV DNA levels. The potential clinical implications of such donations were highlighted by a Japanese report of a case of transfusion-transmitted HBV from an OBI donor who, at the time of the implicated donation, was anti-HBc reactive with an anti-HBs level of 29.6 IU/L but without detectable HBsAg or HBV DNA [18]. In addition, a European look-back study indicated that products from OBI donors may have an overall transmission rate of 28% with rates varying between different components -24% for red blood cells, 51% for platelets, and 85% for fresh frozen plasma [3]. Besides the anti-HBc reactive alone donations, we also observed 163 anti-HBc Total reactive donations with anti-HBs positive < 100 IU/L which could be considered as at the risk of HBV transfusion transmitted infection in the recipients. This highlights the need to further investigate the extent of this risk and effect on the blood supply inventory before considering anti-HBs and anti-HBc screening of the donations to increase the blood safety in the country.
The study also observed high percentage of anti-HBc total reactive in northern RBCs such as Chiang Mai (32%) and Nakornsawan (29.5%) RBC which is consistent with the 2018 National Disease Surveillance Report No. 506 [19] reporting highest HBV prevalence (18.36 per 100000) in the Northern Thailand. Lower percentages were observed from southern RBCs such as Songkhla (14%) and northeast RBCs such as Nakornracharisma (14.5%) and Ubonrachthani (15%), while central Thailand showed higher percentages (NBC 19.3%, Lopburi 23.5%). Thailand has included the HBV vaccine in their expanded program of immunization (EPI) since 1992. In 2017, after 25 years of HBV vaccination through the EPI program, a 99.0% HBV vaccine coverage was reported for the whole country. The variation of anti-HBc reactive percentages could be related to the variable level of access to health care services over the last three decades. Besides that, increased globalization and tourism in some parts of the country could be the potential factors for higher reactive rates in central and northern parts of country.
In summary, we have provided the first report of anti-HBc prevalence among Thai blood donors based on the donations collected from NBC and 12 RBCs. Our results demonstrate the value of anti-HBc testing for the detection of seropositive OBI cases even in a highly endemic country like Thailand. In our study donor population, we found a high prevalence of anti-HBc reactive donations which could potentially be recovered or chronic HBV cases or false reactive but the possibility of seropositive OBI cases with very low HBV DNA levels cannot be ruled out. The inclusion of anti-HBc in our testing algorithm could be pivotal in identifying OBI donors with intermittently detectable HBV DNA. The incremental identification and deferral of OBI donors will further reduce the residual risk of HBV infection in Thailand, which is currently estimated to be approximately 736 in 100,000according to the 2017 TRCS Annual report. However, further investigation is warranted to explore the risks involved for the recipients in terms of infectivity leading to post-transfusion hepatitis. Availability of safe blood is important, and it is the duty of the Transfusion community to continuously explore the risks involved for donors and patients exposed to the so far uncovered population of occult carriers of a virus affecting globally close to a billion individuals.
The authors declare that they have no conflicts of interest.
This research was supported by a grant from Abbott Laboratories, Chicago.
All authors have contributed to the generation of the data (protocol development, sample collection, sample testing, confirmatory testing), provided input into the development of the manuscript, and assisted in its review until the final iteration.