Candida species, a group of opportunistic infection-causing microorganisms, has shown an increasing pattern of resistance against certain antifungal drugs through time. Thus, this retrospective study was conducted to describe the antifungal resistance of Candida species isolated from a tertiary hospital in Bacolod City, Philippines from July 2017 to July 2018. A total of 184 Candida species were isolated from clinical specimens with C. albicans (61%) having the highest frequency followed by C. tropicalis (15%). Most of these Candida species were isolated from older adults (78.26%) and mid adults (15.76%). Additionally, Candida species were frequently present in respiratory specimens (69.02%) and urine specimens (20.65%). Moreover, resistance among the fungal isolates was noted against fluconazole and voriconazole with least resistance to micafungin and caspofungin.
Candida species, Antifungal resistance, Bacolod City
Candidiasis remains as one of the top 5 healthcare-associated bloodstream infections in the world and still causes high mortality rates [1]. It covers a wide range of diseases, from mild clinical infections to invasive and even disseminated forms [1]. In humans, Candida species colonizes the skin, oropharynx and lower respiratory, gastrointestinal, and genitourinary tracts [1]. It has the ability to form biofilm and produce hydrolytic enzymes that disrupt cell membranes which in turn promote its spread in the host resulting in infection [2,3]. Though considered as normal flora, some species can cause opportunistic infections such as that of Candida albicans, Candida glabrata, Candida tropicalis, Candida parapsilosis, Candida krusei, Candida guilliermondii, Candida lusitaniae, etc. [4-6].
In the Philippines, candidiasis constitutes to about 80.40% of the fungal infections in 2016 [6]. Type of infections includes recurrent vulvovaginal candidiasis, oral and esophageal candidiasis, candidaemia, and Candida peritonitis. It was also the leading cause of consultation at the Dermatology Out-Patient Section of the Department of Health-Research Institute for Tropical Medicine. As of this time, there are only four (4) classes of drugs that are available for systemic treatment of candidiasis: azoles, polyenes, echinocandins, and pyramidine [7]. The incidence of Candida infection is increasing, but the choice of appropriate antifungal drugs is limited [5].
Currently, the Antimicrobial Stewardship is being implemented in the country and it has guidelines for the use of antimicrobial drugs, including antifungal drugs. However, variations of treatments may always be present among health institutions or regions [8]. It is, therefore, imperative to monitor epidemiological data among hospitals or regions to establish effective control measures [3] to support institutional, regional, and even national guidelines for empiric treatment [8].
The study was based on the paper of Tan, et al. [8], Lima, et al. [3], and Bhattacharjee [9]. This retrospective observational study was performed in the microbiology section of a tertiary hospital in Bacolod City from July 2017 to July 2018.
Data for this study were taken from the records of patients in the Microbiology Section of a tertiary hospital in Bacolod City. All patients with culture results of Candida species may it be due to colonization or infection were included. Acquired data include the patients' accession number, age, sex, kind of specimen, date of laboratory submission, identified isolate, and its susceptibility test results. In compliance with the Philippine Data Privacy Act of 2012, names and personal information were not taken to keep patients' identity confidential.
Permission for data acquisition was obtained through the approval of the Medical Director of the tertiary hospital. Additionally, the study was given an exemption status by the Colegio San Agustin Bacolod Research Ethics Committee on September 18, 2018.
The laboratory isolated the Candida species through routine culture methods using Blood Agar Plate and Chocolate Agar Plate. Suspected yeast colonies were gram stained and purified by sub streaking to a new blood agar plate. Purified colonies were then identified using the Vitek Compact 2 instrument with software version 8.01 following manufacturer's recommendation and as described in the study of Kaur, et al. [10]. The instrument is considered to be an accurate method for the identification of medically important yeast and yeast-like organisms [11,12]. The same instrument was used to determine the antifungal susceptibility of the isolates. Additional tests were performed that include germ tube assay, urease production, and growth at 45 °C.
Candida species that are phylogenetically related or phenotypically indistinguishable were reported as a complex. This include C. glabrata complex (C. glabrata, C. bracarensis and C. nivariensis), C. haemulonii complex (C. haemulonii, C. duobushaemulonii and C. haemulonii var. vulnera), C. parapsilosis complex (C. parapsilosis, C. orthopsilosis, C. metapsilosis and Lodderomyces elongisporus), C. rugosa complex (C. rugosa and C. pseudorugosa) [13] and C. ciferri complex (C. allociferii, C. mucifera and C. ceferii) [14].
However, according to CDC [15], Vitek Compact 2 with versions before 8.01 has its limitation for it can misidentify rare Candida species like C. auris as C. haemulonii or C. duobushaemulonii. Though the laboratory has the Vitek Compact 2 software version 8.01, all Candida species identified as C. haemulonii and C. duobushaemulonii will be forwarded to a reference laboratory for confirmation of identity. Candida albicans ATCC 90028 was used as the control for identification and in-vitro susceptibility testing of Candida isolates.
Interpretation for susceptibility testing was made in accordance to the guidelines stipulated in the M60 Performance Standards for Antifungal Susceptibility Testing of Yeast [16] and in the Descriptions of Medical Fungi [13].
For statistical analysis, the WHONET software version 5.6 was used to determine the frequency of isolates, percent resistance, and confidence intervals. Furthermore, inferential statistics such as independent T-test was also used to compare resistance between C. albicans and non-albicans Candida.
A total of 184 isolates were identified as Candida species using Vitek Compact 2 instrument, all of which had more than 95% excellent identification. Patient demographics with Candida infection are presented in Table 1 and Table 2.
Table 1: Frequency of Candida Infection as to Age Group and Sex. View Table 1
Table 2: Frequency and Distribution of Candida species According to Age. View Table 2
Isolates were collected from 184 non-repetitive patients. The most frequent among these isolates were C. albicans (62%), C. tropicalis (15%) and C. cefirrii complex (10%). Out of the 184 Candida species isolated, 127 (69.02%) came from respiratory specimens (sputum and tracheal aspirate), 38 (20.65%) from urine specimens, 14 (7.61%) from blood, and 5 (2.72%) from vaginal discharges. Sample site and distribution of Candida species are listed in Table 3.
Table 3: Candida species Isolates Listing Summary. View Table 3
To determine the percent resistance of an organism, at least 30 isolates is needed. In this study, however, it is noted that individual species of non-albicans Candida did not reach the cut-off. It was therefore decided in this study to have it grouped into two: C. albicans and non-albicans Candida.
Generally, it is noted that Candida species had the highest resistance to voriconazole with 8.4% (CI: 4.9-13.7). When grouped as to C. albicans and non-albicans Candida, the highest resistance is still observed in voriconazole with 7.4% and 10% (CI: 5.6-18.2, 7.4-25.2) respectively. On the other hand, the lowest resistance for both groups was observed in caspofungin and micafungin with 0% (CI: 0.0-4.1). Resistance of the Candida species is shown in Figure 1.
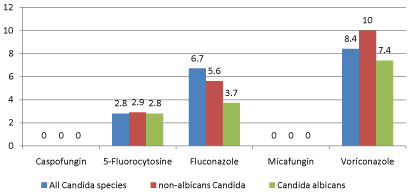 Figure 1: Antifungal resistance of Candida species .
View Figure 1
Figure 1: Antifungal resistance of Candida species .
View Figure 1
Although the resistance of each non-albicans species were not shown because its numbers did not reach 30, it is noteworthy to mention that in this study C. parapsilosis complex, C. glabrata complex, C. lusitaniae, and C. dubliniensis did not show any resistance to the tested antifungal drugs. However, 3% of C. tropicalis was noted to be resistant to fluconazole, while C. ciferrii had shown resistance to fluconazole and voriconazole with 42.1% and 38.9%, respectively.
Using independent t-test, this study observed that there was no significant difference on the percent resistance of Candida species to antifungal drugs, either grouped as C. albicans or non-albicans Candida with a p-value 0.45.
Frequency on Candida infection was most notably observed on respiratory specimens and predominantly in older adults. Aside from the environmental and cultural factors [17], the most probable reasons for this condition are host-related factors such as underlying malignancies, immunosuppressive diseases, hematopoietic stem cell or organ transplantation, frequent use of wide-spectrum antibiotics, invasive interventions, chemotherapy, parenteral alimentation, use of internal prosthetic devices, natural modification of the immune system [3], age, neutropenia, and deteriorating clinical conditions due to underlying disease [1,18].
Candida species have many virulent attributes including hydrolytic enzymes such as proteinases, phospholipases, and lipases. Candida species can also produce biofilm, considered as the most pathogenic trait, which result in treatment failures and recurrent infection [19]. It has also the ability to generate the filamentous form that is important to tissue invasion; however, these are dependent to environmental factors and type of species [2].
Majority of the isolates were identified as C. albicans with a frequency of 61%. This is because C. albicans is the most common and most pathogenic [20] among Candida species [21] worldwide [22]. The findings of this study are similar to the study of Menezes [23], Bhattacharjee [9], and Lima [3]. Further noted in other studies, non-albicans Candida were starting to rise in numbers [9,24] and seen as a suggestive emergence of important pathogens.
Normally, the sources of these Candida species are the patient's own microbiota in the gastrointestinal tract. Endogenous type of infection results from the disturbance of the normal microbial antagonism such as overuse of antibiotics that will lead to the overgrowth of the organism and can reach other sites by translocation [25]. Neither C. auris nor Candida species that can be mistaken as C. auris such as C. haemulonii or C. duobushaemulonii were isolated.
Candida species were mostly isolated from respiratory specimens because they have been considered as one of the commensal constituents of normal human oral microbiota, with minimum significance when detected in respiratory specimens [26,27]. Candida species colonize the mouth of both healthy and unhealthy individuals. Respiratory specimens, specifically sputum, get contaminated as it passes through the mouth during expectoration [26]. This then indicates that Candida species detected from respiratory specimens must not always be considered as cause for lung infection [28]. However, thorough deliberation must also be taken before considering Candida species as an innocent colonizer in the respiratory tract because it has a beta-glucan on its cell wall that acts as a lung pro-inflammatory agent causing macrophage and neutrophil dysfunction. Additionally, airway colonization is also associated with pulmonary inflammation and consequent cellular immune dysfunction, prolonged duration of mechanical ventilation and increased mortality [26,28]. Moreover, the biofilm producing capacity and fungal-bacterial cross-talk should not also be neglected although published clinical data based on this theory to consider Candida species as the true pathogen is still lacking [26,28]. Therefore, physicians' ultimate role is critical in assessing the Candida species as an etiologic agent of pneumonia.
It is also noteworthy to mention the frequency of C. ciferri complex that constitute 10% of the total isolates. With the current available literatures, C. ciferrii is rarely isolated or an infrequent type of Candida species that is known to cause superficial pathogenicity [29-31]. Some papers have documented that C. ciferrii was able to cause blood stream infections [29-31], pulmonary infections [30,32], gangrenous infections [30], onchomycosis [33] and urinary tract infections [14]. Similarities among these infected patients include co-morbidities such as COPD, age, diabetes, malnutrition, malignancy, other chronic diseases, and immunosuppression [31,32]. As observed in this study, majority of the C. ciferrii were isolated from respiratory specimens, and that patients are mostly on the advanced age groups that can be assumed to have similar co-morbidities. As to the source of C. ciferrii, it can also be understood that the infection is a result of translocation of the patient's own microflora.
The urine specimen was also noted to show high frequencies of Candida species because they are likewise commensal of the genitourinary tract [34]. Even so, there are several risk factors that contribute to candiduria which include instrumentation, prior surgical procedure, recent use of antibiotics, advanced age, sex (female), diabetes mellitus, immunosuppressed therapy, and prolonged hospital stay [35]. Moreover, the presence of Candida species in urine could possibly imply contamination [20] unless supported by pyuria after ruling out coexistent bacteriuria, or mechanical injury to the genitourinary tract [35].
In this study, out of 184 cases, only 5 (2.72%) Candida species were isolated from the blood. Though low in number as it may seem, candidemia has attributable mortality of 15-35% for adults and 10-15% in neonates [25]. Species isolated from this study are similar to those of Guinea, et al. [25], Tellapragada, et al. [19], Tan, et al. [8], and Tsai [36]. Mortality due candidemia, however, can be prevented through proper treatment, no delays in the initiation of treatment and by using active agents [25].
Fungal infections, especially by opportunistic species like Candida have been considered as an increasing problem among older adults because older patients may have received transplants, underwent aggressive treatment for malignancies, taking immunosuppressive drugs for dermatologic or rheumatic diseases and other age-related physiological changes [22,37]. Additionally, this age group is at risk for fungal infection when they underwent frequent and prolonged hospital treatment. Moreover, renal failure, total parenteral nutrition, central intravenous catheters, broad-spectrum antibiotic treatment, and prior surgical procedures enhance the risk of Candida infection [37]. Candidemia has become a marker for a poor outcome in older adults with multiple underlying and lengthy ICU stay [28]. These findings were similar to the findings of Yapar1 and Kaur, et al. [18].
The resistance of the isolated Candida species, albicans or non-albicans to antifungal drugs was noted to be almost the same with the p-value of the t-test of 0.45, which is more than the 0.05 mark. The resistance was seen in fluconazole and voriconazole because these are the most frequent drugs used in treating invasive candidiasis, as prophylaxis or of extensive use [18]. This may lead to cross-resistance of either fluconazole or voriconazole and to other azole drugs [7] due to the previous exposures [5].
The preferred agent for the treatment of yeast infections has always been azoles, specifically fluconazole, since it is safer than Amphotericin B [37,38]. Its use, however, may be limited, especially fluconazole because of the increasing prevalence of Candida species with acquired or intrinsic resistance [22]. As observed in this study, resistance to azoles specially voriconazole was higher compared to the other antifungals while echinocandin drugs were noted to be unrivaled. Overall resistance to voriconazole in this study is 8.4%, which is lower compared to the study of Marak, et al. [39] in India 18.88%, and with Tasneem, et al. [40] in Pakistan 14.61%. As with fluconazole, this study noted resistance of 6.7%, which is also lower than that reported in Pakistan [40] with 18.26%, Nepal with 20% [41], and India with 22% [39].
Reasons for azole resistance may be due to overexpression of drug efflux pump, intrinsic resistances, presence of point mutations in ERG11, increased expression of ERG11, or ability of the yeast to grow with altered cell membrane sterols [4] or alteration of sphingolipid composition [42]. Some Candida species have certain characteristics for antifungal resistance. Such that, generally C. tropicalis and C. parapsilosis are both susceptible to azoles [43], C. parapsilosis and C. guillermondii have decreased susceptibility to echinocandins [14] while C. glabrata is intrinsically resistant fluconazole [33]. High level of resistance toward azoles is also known for C. krusei, C. insconspicua, C. rugosa, and C. norvegensis [44]. Though some Candida species are intrinsically resistant to some antifungal drugs, some may still appear susceptible to it because of method variation, mutation or low levels of resistance expression.
Furthermore, the result of this study showed drug resistance of below the 20% cut-off, thus, empiric therapy using any of the antifungal drugs mentioned in this study is still warranted to be effective as of this time. This also gives implications for the growing antimicrobial stewardship efforts of the institution. Moreover, the diagnosis of true Candida infection is imperative prior to administration of antifungal agents to prevent overtreatment.
In the Philippines, the data for antifungal resistance is very scarce as of this time. Also, certain limitation of this study is that it has only 184 isolates and was only tested to 5 antifungal drugs because of the availability of the service in the area and laboratory capability. It did not also specify that all the isolates were noted to be the exact causative agent of the disease or colonization. However, despite the fact that it is a retrospective type, it also gave a baseline data for the frequency of Candida species isolated and its resistance to antifungal drugs. Studies with a greater number of isolates and antifungal drugs may be needed in the future.
This study revealed C. albicans as the most frequent isolate from the different clinical specimens received for examination in Bacolod City, Philippines. It was further noted that Candida species showed resistance to common antifungals used for systemic infection like fluconazole and voriconazole. Considering the changing patterns of infections, clinicians need to consider the result of this study to minimize antifungal resistance. It is recommended that closer monitoring of Candida species distribution and susceptibility to antifungal drugs be made to optimize treatment and outcome.
The authors would like to thank the comments and suggestions of Dr. Greg Ryan Gerongano, Dr. Reed Aaron Cordova, Dr. Rommela Ruiz-Tiplez and Ms. Jo-Anne Lagotang. We would like also to thank Ms. Jessamine Calma for helping us with the statistical analysis.
ACJ, JPTL, GBDR made the design and plan of the study; JFNVG and FRAB did the literature review. ZCDR, ZIPB, JFNVG took the preparations for ethics review; ACJ and JPTL for the data collection; ZCDR, ZIPB, and MDBT performed data analysis and interpretation; ZCDR, ACJ, and GBDR wrote the first draft of the paper; All authors assisted, approved and agreed with the final manuscript.
The authors declare no conflict of interest.