Methicillin resistant Staphylococcus aureus (MRSA) ventilator-associated pneumonia (VAP) is increasing in prevalence. Treatment of VAP has moved toward ensuring patients are adequately covered for MRSA and other MDROs while balancing the need for antimicrobial stewardship and appropriateness of empiric coverage of these organisms in the setting of increasing resistance rates. The objective of this study was to identify the incidence of and risk factors for MRSA VAP in surgical intensive care unit (SICU) patients as a means to better identify patients at risk who would benefit from MRSA coverage empirically.
This was a single-center, retrospective risk factor analysis of adult SICU patients, comparing patients with MRSA VAP to those with VAP due to alternative pathogens. Primary outcomes were incidence of MRSA VAP and risk factors associated with MRSA VAP. A multivariable logistic regression model was performed to identify risk factors for MRSA VAP.
Of 140 included patients, MRSA VAP occurred in 31 (22.1%). Fewer patients in the MRSA group were in septic shock (6.5 vs. 23.9%, p = 0.04) or hemodynamically unstable (16.1 vs. 34.9%, p = 0.03) at the time of VAP diagnosis. A lower proportion of MRSA patients had antibiotic exposure prior to VAP (58.1 vs. 78.9%, p = 0.03). Female sex was associated with a 2.3-fold higher risk for MRSA VAP and history of MRSA with a 4.2-fold higher risk, while patients with prior antibiotic exposure were 60% less likely to develop MRSA VAP.
Ventilator-associated pneumonia due to MRSA was common among this SICU cohort. A history of MRSA infection and lack of prior antibiotic exposure may be useful factors to aid in selection of patients appropriate for empiric MRSA therapy. Risk factor analysis may be difficult in this population due inability to control for all patient-specific variables. Future risk factor studies investigating specific MDRO VAPs and the role of dysbiosis are needed to determine the appropriateness of empiric broad-spectrum therapy.
The incidence of ventilator-associated pneumonia (VAP) remains high in the intensive care unit (ICU) and is associated with prolonged length of mechanical ventilation and increased hospital length of stay [1]. Methicillin-resistant Staphylococcus aureus (MRSA) and other multi-drug resistant organisms (MDROs) have become increasingly prevalent as causative pathogens for pneumonia, with MRSA found to be responsible for 15% of VAP in a survey of 59 US hospitals [1,2]. With increasing antimicrobial resistance, clinicians are often forced to use increasingly broad coverage empirically to ensure appropriate and timely antimicrobial therapy. As a measure of antimicrobial stewardship, a greater emphasis on the identification of patients appropriate for empiric therapy against MDROs is necessary in order to reduce unnecessary exposure to antibiotics, the development of antibiotic resistance, and other antibiotic-related complications.
Known risk factors for MDR VAP are outlined in clinical practice guidelines, however they are not specific to MRSA or to an ICU sub-population, particularly the SICU [1]. Previous factors associated with increased risk of MRSA infection from any source among ICU patients have included ICU length of stay, central venous catheter (CVC) insertion, previous antibiotic use, and the presence of more than 2 patients with nasal colonization in the same ICU at the same time [3]. Higher APACHE II score on admission, receipt of any antibiotic before VAP, and pleural effusion on X-ray have been identified as risk factors specifically for MRSA VAP in a mixed ICU population [4]. A small single center study by Lollar, et al. described risk factors for early VAP due to MRSA specifically in SICU patients at a single institution, however the cohort of patients with early MRSA VAP was very small and the sample size severely limited the ability to identify risk factors [5]. The objective of this study was to identify the incidence and risk factors for ventilator-associated MRSA pneumonia in SICU patients with VAP in order to better identify those patients who may benefit from including MRSA coverage (i.e. vancomycin) empirically.
This was a single-center, retrospective risk factor analysis of adult SICU patients that were selected for lower respiratory culture between January 1, 2013 and August 31, 2016. All patients were admitted to The Ohio State University Wexner Medical Center (OSUWMC), a tertiary, academic referral center and Level 1 trauma center. To meet inclusion criteria, patients must have been diagnosed with VAP during SICU admission. VAP was defined as a positive lower respiratory culture collected > 48 hours after endotracheal intubation [1]. A positive lower respiratory culture was defined as a quantitative lower respiratory culture isolating > 10,000 colony forming units/milliliter (cfu/ml) of a pathogenic bacterial organism(s). Patients with polymicrobial cultures were included. All patients requiring endotracheal intubation during the study period had standard endotracheal tubes without silver or antimicrobial coating. Respiratory cultures used for diagnosis of VAP were obtained either by bronchoscopic bronchial alveolar lavage (BAL) or non-bronchoscopic BAL (aka mini or blind BAL). This method of sampling is standard practice in the surgical ICU at our institution for all cases of suspected pneumonia in ventilated patients in response to clinical and diagnostic criteria. Microbiologic identification of bacteria for respiratory culture was done using standard microbiological testing combined with the use of matrix-assisted laser desorption ionization time of flight - mass spectrometry. Susceptibility testing was performed using the MicroScan Walkaway System® (Siemens Healthcare Diagnostics, Inc., Deerfield, IL). Susceptibilities were determined using Clinical and Laboratory Standards Institute (CLSI) interpretative criteria. Exclusion criteria included no requirement for mechanical ventilation or mechanical ventilation for < 48 hours prior to culture, respiratory culture by sputum or tracheal aspirate, pregnancy, or incarceration. If the patient had multiple VAPs during their SICU admission only the first episode of VAP was included for risk factor analysis, and causative pathogens of that first episode were what dictated group allocation. Standard infection control protocols for the hospital during the study period included cleaning of patient rooms with a quaternary or phenolic cleaner, cleaning of patient rooms with bleach for patients with "enteric" precautions and for all terminal cleaning of rooms, and use of contact precautions (i.e. requirement for gowns and gloves) for patients with a known history or current infection with an MDR pathogen. Informed consent was waived due to the retrospective nature of the study, and this study was approved by The Ohio State University Institutional Review Board.
Based on internal data, an estimated 237 surgical ICU patients developed VAP during the study time period, with an incidence of MRSA of approximately 22%. This sample size and event rate provided at least 80% power to detect a dichotomous risk factor with an odds ratio for MRSA pneumonia of 2.23 or greater (assuming the risk factor appears in 50% of the sample) or a continuous risk factor with an odds ratio for MRSA pneumonia of 1.55 or greater for a one standard deviation increase in the risk factor, at a significance level of α = 0.05.
Patients with MRSA VAP as defined by the isolation of MRSA from a lower respiratory culture at the time of their first VAP during an ICU admission of their index hospitalization were included in the 'MRSA VAP group'. Patients who did not have MRSA isolated from a lower respiratory culture at the time of their first VAP were included in the 'Non-MRSA VAP group'. If a patient had a polymicrobial culture result that included MRSA and a non-MRSA pathogen they were included in the 'MRSA VAP group'. The primary outcome was the incidence of MRSA VAP compared to non-MRSA VAP. Secondary outcomes included risk factors associated with MRSA VAP, total duration of mechanical ventilation, hospital and ICU length of stay, and mortality.
Data was collected retrospectively from the electronic medical record and included patient demographics, reason for hospital and ICU admission, admitting service, Sequential Organ Failure Assessment (SOFA) score at ICU admission, Charlson Comorbidity Index and relevant comorbidities including asthma, COPD, immunosuppression (defined as receiving active chemotherapy treatment or the equivalent of 20 mg of prednisone daily), and chest wall injury or trauma. Risk factors for MDRO VAP (prior IV antibiotic use within 90 days, septic shock at time of VAP, ARDS preceding VAP, hospitalization for ὅ 5 days prior to VAP, acute renal replacement therapy prior to VAP), intravenous drug use, history of MRSA from any source or MDRO infections as documented within the electronic medical record (defined as infection due to microorganisms that are resistant to one or more classes of antimicrobial agents) [6], chronic open wounds, and history of skin abscesses or cellulitis were also collected. At the time of each positive BAL meeting criteria for VAP, the following characteristics were collected: time from hospital and ICU admission to VAP, time from intubation to VAP, SOFA score, whether the patient was on TPN, required chest tube or catheter placement, the presence of any chronic lines and whether the line was placed prior to admission, active order for a proton-pump inhibitor and the ventilator bundle (activity order for head of bed elevated > 30 degrees, mobility orders, chlorhexidine), pleural effusion on chest x-ray, as well as vitals and laboratory data. For each positive respiratory culture, the organism(s) isolated and susceptibilities were recorded, as well as gram stain results, empiric antibiotic coverage for MRSA, duration of MRSA coverage, and antibiotic exposure prior to VAP.
Patients with VAP were categorized as MRSA or non-MRSA based on the earliest identified incidence of VAP. Patient characteristics are reported as counts and percentages for categorical variables and median with interquartile range (IQR) and/or mean and standard deviation for continuous variables. Patients with MRSA and non-MRSA were compared using Fisher's exact test for non-ordered categorical variables, Jonckheere-Terpstra tests for ordinal variables, and Wilcoxon rank-sum tests for continuous variables. A multivariable logistic regression model for MRSA pneumonia was then fit using all variables significant at the α = 0.05 significance level in univariate testing. Variables with p-values greater than 0.05 were sequentially removed from the model to reduce the number of covariates. Predicted probability of MRSA was estimated for each combination of model coefficients. No adjustments were made for multiple testing. All statistical analyses were performed in SAS version 9.4 (Cary, NC).
A total of 366 respiratory cultures were evaluated. Reasons for culture exclusion were intubation < 48 hours (42.9%), sputum or tracheal aspirate sample (11.9%), recurrent or relapsed VAP within the same SICU admission (23.9%), admission location other than SICU at the time of VAP (10.2%), < 10K CFU on respiratory culture (8%), and protected populations (3.1%) (Figure 1). One hundred and forty patients had BALs completed that were eligible for inclusion, with 31 patients (22.1%) in the MRSA VAP group and 109 patients (77.9%) in the non-MRSA VAP group. Groups were similar at baseline in terms of admission location and reason for ICU admission, however there were significantly more females in the MRSA VAP group as compared to non-MRSA VAP group (Table 1). The majority of patients in both groups were admitted to the Acute Care Surgery service (22 patients [70.9%] MRSA vs. 64 patients [58.7%] non-MRSA), with the Burn service being the next most common admitting service, following just under 13% of patients in each group. Both groups had very few patients considered to be immunosuppressed or with a history of IV drug abuse. Patients in the MRSA VAP group had a higher proportion of patients with a history of MRSA infection or history of infection due to a MDRO. No differences were observed in Charlson Comorbidity Index or total SOFA scores on ICU admission. Five patients (16.1%) in the MRSA VAP group had a tracheostomy compared to 7 patients (6.4%) in the non-MRSA VAP group. For patients in the non-MRSA group, the organisms most prevalent for VAP for Enterobacteraciae (32.1%), Pseudomonas aeruginosa (29%), and SPACE organisms (22.9%) (Table 2).
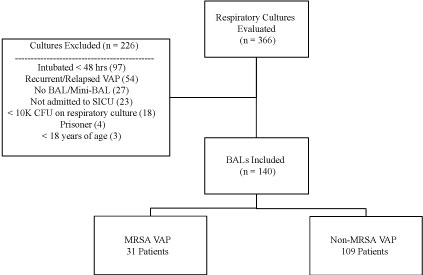 Figure 1: Study Consort Diagram.
Figure 1: Study Consort Diagram.
BAL: Bronchial alveolar lavage; CFU: Colony forming units; MRSA: Methicillin-resistant Staphylococcus aureus; SICU: Surgical intensive care unit; VAP: Ventilator-associated pneumonia.
View Figure 1
Table 1: Patient Characteristics for MRSA and non-MRSA Groups. View Table 1
Table 2: Summary of Organisms for MRSA and Non-MRSA VAP Groups. View Table 2
Risk factors previously defined in clinical practice guidelines as well as those outlined as potential risk factors in the literature are summarized in Table 3 for the MRSA and non-MRSA VAP groups. Patients in both groups received similar VAP prevention measures (i.e. chlorhexidine rinses, elevated head of bead, activity orders for mobility) and no difference was seen in the use of proton-pump inhibitors. Although SOFA scores on ICU admission were similar between groups, patients in the MRSA VAP group had lower SOFA scores at the time of VAP diagnosis. For the specific elements of the SOFA score at time of VAP diagnosis, there were fewer patients with an elevated bilirubin (1 (3.2%) vs. 24 (22%), p = 0.02) and fewer with hemodynamic instability (5 (16.1%) vs. 38 (34.9%), p = 0.03) in the MRSA VAP group. In addition, a lower proportion of patients in the MRSA VAP group met criteria for septic shock at the time of VAP diagnosis compared to the non-MRSA VAP group. A lower proportion of MRSA VAP patients had antibiotic exposure prior to their first VAP during the index SICU admission (Table 3), with 41.9% of MRSA VAP patients vs. 64.8% of non-MRSA VAP patients receiving anti-MRSA therapy, and 45.2% of MRSA VAP patients vs. 66.1% of non-MRSA VAP patients receiving anti-pseudomonal therapy. Groups were similar in exposure to clindamycin, fluoroquinolones, and alternative antimicrobials. At the time of VAP, 87 patients (79.8%) within the non-MRSA VAP group were initiated on anti-MRSA therapy empirically. Of those, 46 patients (52.9%) received linezolid and 41 patients (47.1%) received vancomycin.
Table 3: Process of Care Variables for MRSA and Non-MRSA VAP Groups. View Table 3
There were no significant differences in hospital or ICU length of stay or in the duration of mechanical ventilation, though durations were shorter for all three outcomes in the MRSA VAP group (Table 4). The MRSA VAP group had lower all-cause mortality than the non-MRSA group, although this difference did not reach statistical significance. In the multivariable logistic regression model, female sex was associated with a 2.3-fold higher risk for MRSA VAP and history of MRSA with a 4.2-fold higher risk, while patients with prior antibiotic exposure were 60% less likely to develop MRSA VAP (Table 5). The highest predicted probability for MRSA pneumonia among VAP patients was in females with a history of MRSA and no prior antibiotic exposure at 77%. When looking at males with a history of MRSA and no prior antibiotic exposure, the predicted probability decreased only slightly to 57%. The lowest predicted probability was 11% in males with no history of MRSA and having prior antibiotic exposure.
Table 4: Clinical Outcomes for MRSA and Non-MRSA VAP Groups. View Table 4
Table 5: Multivariable Logistic Regression Model for MRSA VAP. View Table 5
The incidence of MRSA VAP in this cohort of SICU patients was twice what has been described in previous literature examining risk factors for MRSA VAP in SICU patients, though it is in line with reported rates in combined ICU cohorts [3,4]. Selection of empiric antibiotics for VAP must balance the appropriateness of empiric coverage with antimicrobial stewardship to minimize the risks of unnecessary antibiotics. This study identified female sex, history of MRSA, and lack of prior antibiotics as independent predictors of MRSA VAP in SICU patients. In addition, the current study suggests the patients with MRSA VAP had a lower severity of illness at the time of VAP, which was reflected in the trend toward reduced mortality in the MRSA VAP group.
Other studies have utilized timing of VAP occurrence as a method to assist in antimicrobial stewardship, particularly with anti-MRSA therapies. Kashuk and colleagues demonstrated an increased prevalence of late VAP (defined as ὅ 4 days post-ICU admission) as compared to early VAP in trauma patients [7]. MRSA was more common among patients with late VAP (22%) compared to early VAP (2.2%). The authors reasoned the pathogenesis of early VAP was essentially that of a community-acquired pneumonia and therapies should be targeted more toward those organisms. In another small study of SICU patients, Lollar, et al. compared early VAP (defined as 2-4 days from intubation) and late VAP in a cohort with 10.7% incidence of MRSA VAP [5]. Similar to the results by Kashuk and colleagues, a higher proportion of patients with late VAP isolated MRSA as a pathogen (12.3%) compared to the early VAP group (2.8%). There was a substantial increase in the ratio of MRSA as a proportion of VAP starting at day 5, with the rate of VAP due to MRSA increasing to almost 17% by day 6. Median hospital and ventilator day for MRSA VAP were both found to be day 8. In comparing our SICU cohort to these two studies, we found our population to also fall within the late VAP time period, with a median time to from ICU admission to VAP of 9 days and a median time from intubation to VAP diagnosis of 7 days for both groups. Our population had a similar incidence of MRSA VAP as the Kashuk study and almost double that of the Lollar study. Based on these results and those of previous studies, it may be reasonable to withhold empiric anti-MRSA therapy for VAPs occurring in the early setting as a method of antimicrobial stewardship. However, local incidence of MRSA should be considered when making this choice, and due to the high incidence of MRSA at our institution, guideline recommendations would support the use of anti-MRSA therapy empirically. If available, utilization of a VAP-specific or ICU-specific antibiogram can aid in stewardship efforts given the wide variability of patient antimicrobial flora and antimicrobial resistance patterns between ICUs, hospitals, and geographic regions [1].
Presently, only one study has investigated risk factors for MRSA VAP specifically in critically ill surgical patients. In a limited analysis of 3 patients with early MRSA VAP, the abovementioned study by Lollar and colleagues presented a list of 9 risk factors with a negative predictive value of 100%, but a positive predictive value of only 8.6% [5]. Our expanded population of 140 SICU patients (31 with MRSA VAP) demonstrated three alternative factors to be independent predictors of MRSA VAP. Based on these three risk factors, the predicted probability of MRSA VAP was highest among females with a history of MRSA infection and no prior antibiotic exposure at 77%, however males with a history of MRSA infection and no prior antibiotic exposure still had a 57% probability of MRSA VAP, suggesting greater effect from the other two risk factors than from the patient's sex. Males with no history of MRSA infection and prior antibiotic exposure who present with VAP early in their hospitalization appear to be a low risk population in which empiric MRSA coverage may not be necessary. The threshold to utilize MRSA coverage in the absence of the above risk factors and/or in the early VAP period should be dictated by the clinical status of the patient through a thorough evaluation of the risks and benefits of withholding empiric MRSA coverage. Trauma patients with bacteremia secondary to VAP may be a cohort warranting more aggressive empiric therapy given the higher risk of morbidity and mortality in this population [8,9]. Despite the risk factors discovered in this study, more information may needed to guide selection of patients who require empiric MRSA coverage for VAP, and to identify those in whom it would be safe to withhold MRSA coverage empirically.
While many antimicrobial stewardship efforts have focused on minimizing unnecessary exposure to broad spectrum antibiotics through use of gram stain or rapid diagnostics, studies have recently begun to evaluate the role of nasal swabs in guiding anti-MRSA therapy in ICU patients. Chotiprasitsakul and colleagues demonstrated that the negative predictive value of a negative MRSA nasal swab at the time of ICU admission was 99.4% [10]. Of the 11,441 negative nasal swabs in their patient population, 25 patients (0.22%) were deemed to subsequently have a MRSA infection based on the judgement of the treating physician. Despite the low probability of MRSA infection, 4,067 (35.7%) negative nasal swab patients were continued or started on anti-MRSA therapy which equated to 7,364 vancomycin days. Similarly, a smaller study by Langsjoen, et al. reported a negative predictive value of 94% with MRSA swabs obtained on ICU admission in a mixed ICU population [11]. Of the 52 patients with negative MRSA swabs, only 3 (5.8%) later developed MRSA VAP. Smith and colleagues examined the clinical utility of MRSA nasal swabs before or within 48 hours of ICU admission in patients with nosocomial pneumonia and found a negative predictive value of 99.03% [12]. Patients who were continued on anti-MRSA therapy, specifically vancomycin, despite negative MRSA swab results had a significantly longer ICU length of stay. The current study had no standard MRSA screening in place during the study period and therefore MRSA nasal swabs results were not included as a variable in our analysis. During the four-year study period, 40-55% of Staphylococcus aureus infections were due to MRSA, however this was not specific to VAP. Given the emerging evidence for MRSA screening in prior publications and our finding that history of MRSA may play a role in risk for MRSA VAP, MRSA screening may be of value in this patient population to safely guide selection of patients who may not require empiric MRSA coverage when VAP is suspected (i.e. those who screen negative for MRSA).
It is possible that the variables chosen for evaluation in the current study, as well as previous studies, remain too generalized from a population standpoint and do not account for patient-specific factors that may play a greater role in risk for MRSA VAP. A growing area of research and one factor not accounted for historically is the patient's unique microbiota. The mutualistic relationship between a host and the commensal organisms of the gastrointestinal tract has been described in the literature in relation to the role that a person's microbiota plays in immune system regulation and antibacterial defenses. Critical illness itself has been suggested to potentiate the loss of such commensal microbes and lead to the overgrowth of pathogenic bacteria [13]. This dysbiosis can occur as a result of individual or a combination of stressors, including lack of nutrition, blood pressure augmentation, and antibiotic therapies [13,14]. While risk factor analyses can account for some stressors, the exact dysbiosis has not been included to date in evaluation. McDonald and colleagues sought to characterize microbiome changes in 115 ICU patients versus healthy controls by collecting fecal, oral, and skin samples within 48 hours of admission and at ICU discharge or day 10 of ICU admission [14]. Their results demonstrated lower levels of two of the largest groups of gut microbes, increased abundance of pathogenic bacteria in ICU patients, and overall decrease in diversity of microbes; these changes occurred within days of ICU admission and lasted the duration of ICU admission. The loss of protective microbes that encompass a large portion of gut immunity have been shown in mouse models to increase the susceptibility to S. aureus pneumonia [15]. While ICU stressors in general may affect the microbiome during an ICU admission, Zaura and colleagues suggest that antibiotic therapies in particular may affect an individual's microbiome for up to a year [16]. Additionally, antibiotic exposure was associated with an increase in the presence of genes associated with antibiotic resistance in both the oral and gut microbiome. Their results demonstrate that even a single antibiotic course may have long-lasting consequences, and prior antibiotic therapy up to even a year prior could increase the risk of antibiotic resistant organisms. This idea is reflected in the current study, and while prior antibiotic exposure was found to be protective against MRSA VAP the majority of patients with non-MRSA VAP had a MDR gram-negative organism as the causative pathogen. The microbiome and the effect on host immune defenses may play a much larger role in the risk of MRSA VAP than previously thought, and while many of the previously mentioned ICU stressors are often measured as risk factors, perhaps a patient's individual microbiota may be a more specific measure to predict risk of infection by specific organisms in ICU patients.
This study has several limitations worth noting. Limitations include the retrospective nature of the study, and the possible inability to capture all data through a retrospective chart review. Additionally, the single-center design limits generalizability, as results are specific to the microbiology of the patients at this institution. We chose to only include the first episode of VAP for each patient, so results may not be applicable to patients with second episodes of VAP. Generalizability may further be limited by diagnoses made from respiratory cultures obtained only through invasive techniques as not all institutions employ such measures. While we attempted to add to the small pool of data for a surgical ICU population, application of this study would be limited to those patients specifically. Additionally, the SICU at OSUWMC does include burn patients, which some may argue are different than acute care surgery patients in terms of barrier function and antibiotic exposure. We were unable to include the MRSA colonization status of our patients, as nasal swabs are not routinely collected upon admission to our SICU. Finally, due to the retrospective nature of the study, we were unable to discern attributable mortality from all-cause mortality. Further investigation with a larger, prospective study will be needed to confirm the results of this study.
Ventilator-associated pneumonia due to MRSA was common in the SICU population at this institution and occurred in over 22% of patients. This study identified female sex, history of MRSA, and lack of prior antibiotics as independent predictors of MRSA VAP in SICU patients, however alternative variable such as timing of VAP onset and MRSA colonization status of the patient may also be important factors to consider. These results suggest that when considering empiric antimicrobial therapy for suspected VAP in patients admitted to the SICU, patients who are female, lack prior antibiotic exposure and/or have a history of MRSA are at risk for MRSA and should therefore receive empiric MRSA coverage (i.e. vancomycin). Clinical judgement should continue to guide the choice to include MRSA coverage empirically in patients with suspected VAP without these risk factors. Future risk factor studies investigating specific the roles of MRSA screening and dysbiosis are needed to truly determine the appropriateness of empiric MRSA coverage in patients with VAP.
The authors have no financial disclosures or conflicts of interest to report.