Iatrogenic disease represents an ongoing issue for veterinarians where animals manifest with secondary conditions as a result of medical treatment. Similarly, zoonotic disease and specifically resistant zoonotic pathogens represent an ongoing issue for public health safety. As an increasing number of zoonotic microbial species are being recognised as emerging and re-emerging diseases in humans, the issues relating to their antimicrobial resistance becomes more evident. This study reports on two cases of iatrogenic invasive microbial disease in companion animals following previous treatment for dermal conditions. Species identified included Candida krusei, E. coli, MRSA and E. hirae. Furthermore, isolated species both fungal and bacterial were also shown to display antimicrobial resistance. Antifungal resistance in Candida species is an emerging problem particularly as infection with this species has a high mortality rate in immunocompromised persons. Typically, cutaneous infections in companion animals are treated with broad spectrum beta lactamase antibiotics often accompanied with glucocorticoid steroids. This study suggests that more specific diagnostic measures need to be taken to identify the causative agents of infection, to optimise disease treatment and potentially lower the emergence of resistant species.
Zoonotic, Iatrogenic, Resistance, Candida, Mortality
Iatrogenic diseases are secondary diseases which result from the diagnostic and therapeutic treatment of a patient presenting with a primary disease state. The aim of the medical and veterinary disciplines is to treat illness and do "no harm" in the process, however this is often unavoidable as numerous drugs run the risk of adverse drug reactions (ADRs). ADRs are defined by World Health Organization (WHO) as an undesirable response to a therapeutic agent which is toxic, unintentional and which occurs at prophylactic, diagnostic and therapeutic doses. ADRs are classified as Type A (predictable) including side effects, toxicity, super infection, drug interactions and Type B (unpredictable) intolerance, idiosyncrasy, allergy [1]. While these ADRs are commonly recognised and treated, they remain an ongoing problem often resulting in iatrogenic disease. In veterinary medicine, iatrogenic diseases are common with the prescribing of certain drugs. Typically, the treatment of companion animals for mycotic or bacterial infections often requires the prescribing of non-antimicrobial therapeutics such as steroids (prednisolone or trilostane) to elevate symptoms such as pruritus and subsequent alopecia. Prolonged treatment with these immune suppressors may lead to secondary disease of the animal such has iatrogenic Addison's disease or hyperadrenocorticism [2]. In the case of microbial infections of the animal secondary infections may also manifest potentially having antimicrobial resistance (AMR) as a result of continued exposure to therapeutics. The proliferation of AMR in companion animals represents a serious risk to public health safety from zoonotic transmission. Furthermore, microbial colonisation of different and varied host species enables bacteria to proliferate and increase their drug resistance profile leading to multi drug resistance (MDR) via horizontal gene transfer, allowing for a decreased susceptibility to antimicrobial agents in increasing numbers of pathogens [3]. Such MDR bacterial resistance to medically-important antibiotics including extended spectrum beta-lactams (ESBL), carbapenems, fluoroquinolones and/or aminoglycosides, has become more frequent amongst zoonotic pathogens [4]. MDR species, particularly ESBL producing Enterobacteriaceae are often detected in severe cases of infection in companion animals leading to biosafety and disease control issues for the animal and associated personnel [5]. Approximately 1500 recognised pathogenic species are known to infect humans, with 61% of these species contracted via zoonotic transmission [6]. Additionally, pathogenic strains of the zoonotic Candida genus are responsible for 46,000 invasive human infections with a 30% mortality rate [7]. At present, there is a lack of published data available relating to the occurrence of iatrogenic disease and specifically iatrogenic microbial infections in companion animals. Particularly where evidence of the emergence of antimicrobial resistance has occurred in the secondary illness as a consequence of treating the primary condition. Therefore, this study will look at the effect of dermal microbial conditions and subsequent systemic disease on proliferating antimicrobial resistance in companion animals. This report provides some insight into the pathogens associated with dermal and invasive infections in companion animals and may therefore, inform developmental procedures to improve infection control measures. It is believed that this case report will provide valuable information to veterinary practices and promote public awareness to AMR and zoonotic disease.
Study reports on 2 canine patients representing with dermal symptoms of infection, cases were assessed via veterinary therapeutics and microbial diagnostics to determine the causative agents of the disease symptoms. Fungal microbial infections where analysed for levels of resistance of the causative pathogens using antifungal loaded disks on cultured microbial agar plates and cell growth inhibitory activity. Canine patients included in the study had received veterinary treatment and had subsequently manifested with a secondary condition. Exclusion criteria included cases where no link was established between a primary and secondary condition and where no causative agent was identified.
Spayed female terrier exhibiting chronic pruritus for greater than 4 weeks. Erythema evident on the ventral abdomen emitting a strong fusty odour. A round inflamed area of skin also present with a distinct oily discharge in a large area over the pelvis and an oily coat covering the back to the neck. Pruritic, swollen, discharging and painful paws also evident. Preliminary treatment prescribed consisted of antifungal shampoo bi-weekly with malaseb (miconazole and chlorhexidine) (Dechra Veterinary Products, Uldum, Denmark), prednisolone 5 mg daily and oral antibiotics (250 mg cefalexin) twice per day for 3 weeks. Skin scrape for microbial culture using a blunted scalpel blade identified Candida krusei and Escherichia coli (E. coli) cutaneous infection via selective agars (Chromogenic E. coli/coliform Selective Agar, ThermoFisher Scientific, Ireland) and PCR amplification. Approximately 2 months post cutaneous infection, patient presented with pyometra and nephritis, dull, pale, diarrhoeic and foul-smelling urine. Prescribed 10 days of marbofloxacin (2 mg/kg daily) and amoxicillin with clavulanic acid (125 mg) with hypoallergenic renal diet low in sodium, phosphorous and protein adjusting the urine pH to provide a hostile environment to the microbes. Also received rehydrating drip (IV) for the first 3 days. Urine sample and microbial culture identified Candida krusei (as below) and ESBL E. coli via ESBL selective agar (CHROMagar™ ESBL, Cruinn Diagnostics, Ireland), iatrogenic urogenital microbial infection diagnosed, candiduria (Candida species in the urine). For fungal species identification a single colony was selected for amplification using fungal primers ITS1-F 5'-CTT GGT CAT TTA GAG GAA GTA A-3' and ITS4 5'-TCCTCCGCTTATTGATATGC-3' (Sigma Aldrich, Dublin, Ireland) of intergenic spacer regions (ITS) of rDNA. Direct colony PCR was performed in a total reaction volume of 20 µl (17 µl red Taq 1.1x master mix (VWR, Dublin, Ireland) 1 µl ITS1F, 1 µl ITS4 and 1 µl) of selected colony suspension. For bacterial species the same protocol was used with primers UniF 5'- AGAGTTTGATCCTGGCTCAGG -3' and UniR 5'- ACGGCAACCTTGTTACGAGT-3' (Sigma Aldrich, Dublin, Ireland) used for amplification of 16s rRNA gene. DNA amplification, clean-up and gene sequencing of PCR products were completed by Source Bioscience (Waterford, Ireland). Antifungal susceptibility patterns of isolated fungal species to amphotericin, caspofungin and fluconazole was determined using the Kirby Bauer Assay as per the method of Meade, et al. [8], and cell inhibitory activity determined by inoculating a single fungal colony in sabouraud broth containing varying antifungal concentrations over night at 30 ℃, with viable cell numbers determined via a plate count technique. Antifungal testing of both the cutaneous and urogenital strains of C. kruesi via the Kirby Bauer assay (Figure 1) showed that the urogenital strain was more resistant to both fluconazole and caspofungin at concentrations of 2.5 and 12.5 µg/ml. However, for AMPB the urgenital strain showed greater sensitivity than the cutaneous. This pattern also emerged for AMPB when assessing its ability to inhibit the growth of both strains (Figure 2). Caspofungin proved most efficient antifungal (Figure 2) providing the greatest inhibition of cell growth with a 4 log inhibition at 200 µg/ml for the cutaneous isolate. The urogenital strain however, proved much more resistance to caspofungin with a maximal inhibition of 2.1 log at 200 µg/ml. Fluconazole proved the least effective at inhibiting cell growth will a maximal 1.3 log inhibition of both strains at 200 µg/ml. At concentrations below this the urogenital strains again proved more resistant to treatment than the cutaneous. Figure 1 and Figure 2 demonstrate that the secondary kidney fungal infection in this animal had significantly increased antifungal resistance compared to its cutaneous counterpart to both caspofungin and fluconazole.
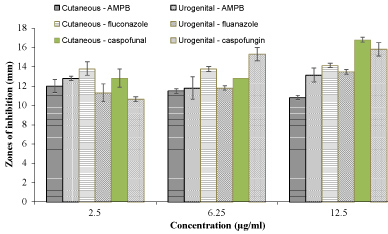 Figure 1: Antifungal susceptibility testing of Candida krusei cutaneous and urogenital isolated species to a range of antifungal therapeutics via the Kirby Bauer assay.
View Figure 1
Figure 1: Antifungal susceptibility testing of Candida krusei cutaneous and urogenital isolated species to a range of antifungal therapeutics via the Kirby Bauer assay.
View Figure 1
 Figure 2: Antifungal inhibitory testing of Candida krusei cutaneous and urogenital isolated species to a range of antifungal therapeutics as log inhibition of cells (cfu/ml) vs. antifungal concentration (µg/ml).
View Figure 2
Figure 2: Antifungal inhibitory testing of Candida krusei cutaneous and urogenital isolated species to a range of antifungal therapeutics as log inhibition of cells (cfu/ml) vs. antifungal concentration (µg/ml).
View Figure 2
Neutered male intermittently treated with prednisolone for environmentally stimulated hypersensitivities for 12 months manifested with severe pruritis, skin flaking, alopecia and areas of purulent discharge, hair follicle infection (pyoderma). Skin scrape displayed demodex colonisation and microbial culture identified non-resistant Staphylococcus aureus, E. coli and Enterococcus hirae bacterial species. Patient prescribed bi-weekly shampooing with malaseb wash and oral ivermectin (anti-parasitic) once daily for 3 weeks with amoxicillin and clavulanic acid (750 mg) twice daily for 2 weeks. Topical amitraz (insecticide) also applied dermally twice weekly for 4 weeks with strict adherence to a hypoallergenic diet. Complete hair loss occurred. Dermatologically, patient appeared recovered with no further symptoms evident accompanied by increased energy levels. Approximately 2 months post cutaneous infection patient spontaneously fell showing signs of neurological disease including incontinence, spasms, slow pupillary reflex, poor response to and recognition of owner while wandering aimlessly with involuntary spasms of hind limb. On clinical evaluation neurological disease was considered most likely due to slow pupillary light reflex and overflow incontinence. Differential diagnosis included neoplasia, abscess, meningitis, haematoma formation or epilepticus, secondary neurological symptoms include secondary hepatic or renal disease. Blood biochemistry, haematology and microbial analysis was performed. Initially prescribed the anti-epileptic pexion (600 mg twice daily) and increased prednisolone (30 mg twice daily) to alleviate symptoms. Haematology showed increased white blood cell count particularly monocytosis with biochemical analysis clear. Final diagnosis bacterial meningitis of haematological origin identified at microbial culture. Bacterial species identified ampicillin, streptomycin and methicillin resistant S. aureus (MRSA) and E. hirae showing resistance to ampicillin, streptomycin and vancomycin. Antibiotic resistance profile established using selective agars (CHROMagar™ MRSA, Cruinn Diagnostic, Ireland) and antibiotic susceptibility disks (Oxoid™ Vancomycin, Oxoid™ Ampicillin and Oxoid™ Streptomycin Antimicrobial Susceptibility Disks, ThermoFisher Scientific, Ireland) as per Schaufler, et al. [9]. Patient prescribed subcutaneous florfenicol (900 mg) 4 ml every 3 days for 4 weeks, with complete recovery of the patient and no reoccurrence of the issue. Diagnosis of iatrogenic dermal pyoderma and subsequent invasive AMR meningitis due to prolonged treatment of dermal hypersensitivity issues with glucocorticoid steroids having immunosuppression activity.
Invasive mycotic infection caused by Candida species represents a significant threat to vulnerable persons particularly neonates, immunocompromised and cancer patients with mortality a high risk. Candida krusei species possess numerous virulence factors such as proteases and hyphae [10] which enable the pathogen to penetrate and cross the dermal layers and enter the systemic circulation resulting in organ mycosis. Furthermore, candiduria arising in critically ill patients should be regarded as an indicator for the possibility of invasive candidiasis. In cases where the immune system of the host is compromised such as patients on prescribe immune suppressors (e.g. prednisolone) or the presence of additional pathogens, breaches of host defences allow for the development of candidemia, with urogenital infection often occurring, as in case study 1. Such predisposing conditions allow for the survival of systemic or locally invasive yeast in sufficient numbers to evade the local or systemic immune response [11]. The canine patient described in case 1 received an initial course of cefalexin a beta-lactam antibiotic and first-generation cephalosporin, for 3 weeks, a therapeutic with no antifungal activity along with a dermatological shampoo and prednisolone. An association between the proliferation of antifungal resistance in fungal species (via activation of the efflux pump) following prolong exposure to antibiotics has been established, and suggests that this may have contributed to the increased resistance of the urogenital isolates to caspofungin and fluconazole seen in this study. Furthermore, the presence of ESBL E. coli in the urine indicates that this species may have gained beta lactamase resistance due to prior exposure to a beta lactam antibiotic. Such pathogenic production of ESBLs is an important resistance-mechanism that is having dire consequences on the treatment of human infections caused by Enterobacteriaceae and is seriously impacting on current antibiotic therapy options [12]. Issues particularly arise as some strains display additional resistant against fluoroquinolones, aminoglycosides, and other classes of antimicrobials [9]. In this case, the ESBL species was not resistant to third-generation cephalosporins or fluoroquinolones which are recognised by the WHO as critically important antibacterial classes for public use. The presence of such resistant pathogens in canines is very significant in terms of its implications and transmission routes for public health safety, as humans and dogs live in such close proximity making it a putative risk factor for ESBL transmission cross species. C. krusei candiduria may have responded well to the renal diet given to the animal once the secondary antibiotic treatment (marbofloxacin and amoxicillin with clavulanic acid) eradicated the ESBL E. coli as acidic pH is known to be favourable for Candida species with alkaline pH decreasing Candida cell proliferation [13]. Studies have reported adjusting urine pH to alkalinity using potassium-sodium-hydrogen citrate eliminated the candiduria in 89% of patients [14]. The resistance of C. krusei to the broadly used fluconazole represents a serious issue to public health safety as it remains one of the five most prevalent causes of clinical yeast infections, contributing to significant levels of morbidity and mortality in immunocompromised persons [7]. Furthermore, the high concentrations of all antifungals needed to achieve inhibition of fungal cell growth, highlights the resistance of this species as these concentrations are not safe for human use. The continued prescribing of prednisolone as seen in case study 2 may have reduced the immune status of the animal and allowed for parasitic skin colonisation with bacterial pyoderma followed by dermal penetration and subsequent systemic infection with AMR species. The presence of MDR Staphylococcus and Enterococcus species is of importance as the resistance profiles of these pathogens are commonly similar to the resistance profiles of the species isolated from human nosocomial infections displaying resistance to aminoglycosides, macrolides, quinolones and tetracyclines [15]. Additionally, Enterococcus species are increasingly recognised as a safety risk due to their role in infectious diseases including bacteraemia, wound infections, endocarditis, meningitis and urinary tract infections coupled with their antibiotic resistance via novel routes of evasion [16]. Staphylococcus aureus remains the most frequently isolated bacterial pathogens in human incidence of skin and soft-tissue infections, pneumonia, septic arthritis, endocarditis, osteomyelitis and sepsis [17]. MRSA isolates being resistant to currently available penicillin's and other β-lactam based therapeutics represent a major contributor to the increasing issues of MRSA infection. Where such infections were once confined to nosocomial outbreaks there has been a huge increase in the incidence of MRSA infections for persons outside of healthcare settings [18]. As such, the zoonotic transmission of Enterococcus and MRSA strains becomes more viable as a route of infectivity in non-hospitalised diseased persons. The decreased ability of the animal to fight infection allowed for the proliferation of multiple species with invasive and potentially fatal results, with iatrogenic meningitis as the most severe consequence. The occurrence of iatrogenic disease following steroid treatment has been previously recognised in cases relating to the adrenal gland activity. Ensuring the administration of an appropriate antimicrobial therapeutic to any patient is important for effective therapy. However, the impact of prescribing antibiotics to animals without identifying the causative agent of infection potentially contributes to the emergence and occurrence of AMR. By doing so, veterinarians may unwittingly be exposing non-resistant species to selective pressure subsequently driving the development or acquisition of AMR. Appropriate infection control practices and measures are essential to prevent the emergence and transmission of ESBL producing bacteria and other MDR pathogenic species.
There is a need for improved surveillance and diagnostics for use in veterinary applications in order to ensure specific targeted treatment of animals and adherence to the One Health approach. As increasing numbers of the population co-habitat with companion animals the risk of zoonosis increases, particularly in terms of re-emerging pathogens. The incidence of AMR in zoonotic pathogens in companion animals is increasing globally due to the overuse and misuse of broad-spectrum antibiotics. There is an ominous and urgent need for regulatory control of the prophylactic and metaphylactic veterinary use of antimicrobial therapeutics. An improved regulatory system will help ensure public health safety and also aid in providing optimised treatment to animals.