Malaria and soil-transmitted helminth infections are morbidity causes in most tropical areas in the world. In Côte d'Ivoire, their association greats a major public health problem and their coexistence is the subject of very few studies. The current study investigated uncomplicated malaria and intestinal helminths co-infection among schoolchildren in Abobo District, Abidjan. This cross-sectional study was conducted with 256 children aged 2 to 15 recruited at the Anonkoua - Kouté Urban and Community Health Centre and at the El-Rapha health centre in the Commune of Abobo in Abidjan. Blood and stool samples were collected from each child. A thick drop examination was carried out on the blood samples whereas the parasitic examination test consisted of a direct microscopic examination, the simplified Ritchie and the Kato techniques. The overall prevalence amounted to 50.3% and Plasmodium falciparum was the only species found. The soil-transmitted helminth infection was found at 15.2%. Co-infestation P. falciparum/intestinal helminth infection was 9.8%. The most found helminth was Ascaris lumbricoides (11.7%). The co-infested subjects had a relatively higher average plasmodia density (23,865 trophozoïtes/µL) than those suffering from malaria only (20,876 trophozoïtes/µL). However, this association was not non-significant when multivariate logistic regression analysis was performed (Adjusted OR = 1.20, p = 0.066).
This study shows a low prevalent of co-infection malaria and Soil-Transmitted helminths and a trend of a higher P. falciparum parasitic density among children infected mostly by A. lumbricoides. The associations between malaria and helminth infections detected in this study were not conclusive and hence needs further investigation.
Malaria, Intestinal helminths infection, Co-infestation, Parasitic density
The parasitic diseases are a major cause of morbidity and mortality in Africa and malaria holds a very important part in Sub Saharan Africa where we find around 90% of clinical cases and more than 80% of deaths [1].
Similarly, the intestinal helminth infections are very prevalent among subjects living in inter-tropical areas with more than 1.5 billion people infected and their association with the plasmodium creates a real public health problem [2]. This indeed suggests the possibility of an antagonism or a synergism between parasites [3]. In subjects carrying many infections at the same time, the body reacts and becomes the place for multiple interactions with implications on the acquisition and development of immunity [4].
It was demonstrated that the infection by Soil-Transmitted Helminths (STHs) could have an impact on the Plasmodium parasitic density and the clinical features of malaria [5]. This co-intestinal helminths and malaria has already been studied in animal and human models [6], and it was reported in human patient of different ages [7].
In the Côte d'Ivoire context, where malaria rages with an upsurge during the rainy season, populations are more exposed to the risk of having simple or severe malaria attacks. The Same populations are highly exposed to intestinal worm portage [8,9]. In these conditions favourable to multi-parasitism, there could be an influence of the helminth infection portage on the expression of malaria. However, few studies were conducted on the occurrence of this co-infection in our context. The current study investigated uncomplicated malaria and intestinal helminths co-infection among schoolchildren in Abobo District, Abidjan (Côte d'Ivoire).
This cross-sectional study was carried out from August 2011 to March 2012 in the Anonkoua Kouté Urban and Community Health Center (N 5°21'24.7", W 4°05'30.7") and at the El-Rapha Health Center in the Commune of Abobo in the North - East of Abidjan (N 5°24'15.9", W 4°01'11.6") the economic capital of Côte d'Ivoire. The climate consists of two rainy seasons: from mid - March to July for the heavy season and from October to November for the small season and two dry seasons: from December to mid-March for the major season and from August to September for the small one. The weather is hot and humid except when the harmattan blows (a dry and cold wind) [10]. The sanitary situation in Abobo is poor and the commune is insalubrious and suffers from a lack of waterworks to evacuate waste waters and rain water thus creating an environment favourable to the development of parasites.
The study was focused on children suffering from uncomplicated malaria received in the Health Center. Inclusion criteria were age from 2 to 15 years, Plasmodium infection detected by microscope examination, axillary temperature ≥ 37.5 °C or a history of fever within the last 24 hours. Children with signs or evidence of severe malaria, low body weight (< 5 kg), signs of malnutrition, repeated vomiting, inter-current infectious disease were excluded because of the high risk of lethality of this malaria form and the difficulty to collect stool samples of these patients [11]. An official approval was obtained from the National Ethic and Research Committee (CNER-CI) before the start of the study. Informed written consent was obtained from schoolchildren parent or legal representative.
A questionnaire was used to collect socio-demographical data from children. Blood and stool samples were collected into sterile for parasitological examination.
After inclusion, patients were subjected to a thick and thin blood films were performed by Giemsa stain for the diagnosis malaria. In the case of Plasmodium trophozoites, parasitemia was determined by counting the number of asexual parasites in 200 white blood cells using leucocyte standard number of 8000/µL [11]. A double reading was performed for all slides. A negative result was declared after checking at least 200 microscope fields.
Stool samples were also collected the same day for intestinal helminths diagnosis using the Kato-Katz method [12]. Two thick smears from each stool sample were microscopically examined to identify intestinal helminths eggs. Test for anaemia detection was not performed.
The data were processed and analysed with STATA 15.0 (STATA Corp., Texas, USA). All the variables noted were described by group. The comparison of the groups was realized by χ2 or Fisher tests and Mann Whitney. The values of p lower than 0.05 were considered as significant. In the multivariate analysis, presence or absence of infection was compared Health Center, age groups, gender and other infections using logistic regression analysis fitted as a generalized linear model and adjusting for possible clustering.
On the basis of signs suggesting malaria, 256 children were included. The patients were aged 2 to 15, averaging 7.5 years old (Standard deviation = 3.4). The age group from 6 to 10 years was the most represented. The sex-ratio was 1.3 (Table 1). The most encountered clinical signs were fever, headaches and chills respectively in the proportions of 86.7%, 85.6% and 71.4%.
Table 1: Distribution of patients according to age and sex. View Table 1
The proportion of positive thick drop examination was 50.8% (IC95%: 44.7-56.9) with a mean parasitemia of 20,870 trophozoïtes/µl of blood. Plasmodium falciparum was the only plasmodia species identified in this study. The patients having a parasitic density higher than 10,000 trophozoïtes/µL of blood were the most observed with a proportion of 54.6%. The patients aged 6 to 10 years presented the highest parasitic density that is more than 10,000 trophozoïtes/µl. However, no significant association was observed between age and the parasitic density (p = 0.57) (Table 2).
Table 2: Intensity of P. falciparum infection, stratified by sex and age. View Table 2
The overall prevalence of soil-transmitted helminths infection was 15.2%. Ascaris lumbricoides and Trichuris trichiura were two STHs found in the current study. Then, the majority of children were infested by A. lumbricoides (11.7%; IC95%: 8.2-16.1), but there was no significant difference (p = 0.66). T. trichiura was also encountered in 3.5% of the cases (IC95%: 1.7-6.3) (Table 3).
Table 3: Overall prevalence of parasitic diseases investigated stratified by sex. View Table 3
The prevalence of the co-infection malaria – soil-transmitted helminths was 9.8% (IC95%: 6.6-13.9) without any statistically significant difference (p = 0.14). The mean parasitic density of P. falciparum in this co-infestation was 23,865 trophozoïtes/µL of blood with extremes of 192 and 95,970 trophozoites/µL. This mean parasitic density was relatively higher than the one of patient having a positive thick drop, not carrying any intestinal parasites, that is 20,870 trophozoites/µl of blood without any statistically significant difference with p = 0.98 (Figure 1). The gender had no influence on this co-infestation (p = 0.43), as well as the age of the children (p = 0.42). The clinical signs most encountered in co-infested patients were fever (87.5%), headaches (87.5%) and chills (66.7%).
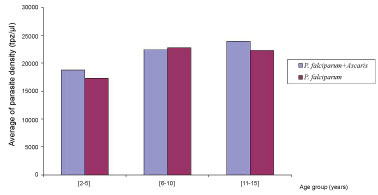 Figure 1: Co-infection Plasmodium falciparum/Ascaris lumbricoides: Plasmodium density by to age.
View Figure 1
Figure 1: Co-infection Plasmodium falciparum/Ascaris lumbricoides: Plasmodium density by to age.
View Figure 1
Outside the age group from 6 to 10 years, all the malaria infested children by A. lumbricoides (7.8%; IC95%: 5.0-11.6) presented a mean parasitic density of P. falciparum slightly higher than the one of children having no intestinal parasites (Figure 2). But the difference observed was not significant (p = 0.63). The number of subjects carrying a P. falciparum and intestinal helminths was higher (44%) when the parasitic density was lower than 10,000 trophozoïtes/µL of blood. Multivariate logistic regression analysis was performed. Variables included in the analysis were age group, sex, malaria infection and co-infection malaria – Soil-Transmitted Infection. Malaria infection occurred more frequently in older children (11-15 years) compared to younger children (2-5 and 6-10 years) and but the difference was significant (p = 0.045). However, this association turned to be non-significant when multivariate logistic regression analysis was performed while adjusting for other confounding factors (Adjusted OR = 1.20, p = 0.066). According to Co-infection malaria and STHs, the association was not statistically significant with analyzed variables. The results of the final model are summarized in Table 4.
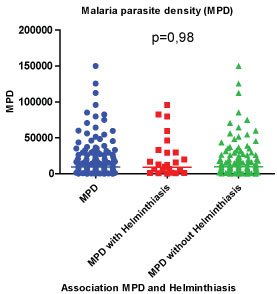 Figure 2: Association malaria parasite density (MPD) and helminthiasis.
View Figure 2
Figure 2: Association malaria parasite density (MPD) and helminthiasis.
View Figure 2
Table 4: Results of multivariate logistic regression analysis showing malaria infection and co-infection malaria - Soil-Transmitted Infection (STHs) with adjusted odds ratios. View Table 4
The study about the influence of certain pathologies associated to malaria is of the utmost importance. Indeed, the occurrence of malaria, and its association with intestinal helminth infection, increases the risk of morbidity in the populations exposed [13]. Half of the study participants were positive for malaria. Côte d'Ivoire is experiencing an epidemiological transition, with the prevalence of clinical malaria currently on the increase, following a significant decline after the introduction of artemisinin-based combination therapies, and intermittent preventive treatment. The disease is responsible for one-third of reported deaths in health facilities in the country [14].
In the current study, only A. lumbricoides and T. trichiura, as soil-transmitted helminths, were found in stool samples. These STHs were reported by others studies as well [15-17].
The prevalence of the co-infestation malaria and soil-transmitted helminths infection observed in our study is lower than the one reported by Faye, et al. in 2008 who reported 29.2% of prevalence [18]. Another study conducted in Tanzania showed a high prevalence of 29% [19]. Our studies do not show a significant association between malaria and intestinal helminth infection contrary to those by Degarege, et al. [20].
In our study, P. falciparum and A. lumbricoides were the predominant parasites found in patients. The co-infested children by P. falciparum and A. lumbricoides presented parasitic densities of P. falciparum slightly higher than those having a positive thick drop, not carrying any intestinal parasites without a statistically significant difference. These results match the ones by certain authors [18]. Likewise, studies conducted in Ghana and Uganda showed that in the case of A. lumbricoides- Plasmodium co-infestation, the Plasmodium parasitic density was also high [21,22]. Nacher suggested that Ascaris infections had a protective effect on particular clinical forms of human malaria, such as cerebral malaria or acute renal failure [23]. Another study led to opposite results, showing a facilitating effect of Ascaris on severe malaria [24].
The presence of intestinal parasites in children suffering from a malaria attack seems to contribute to the increase of the parasitic density [18]. This could also be explained by a decrease of the anti-plasmodium immunity due to the decrease of the anti-sporozoites immunity of type Th1 caused by the helminth infestation [23]. A co-infestation could be responsible for a chronic anaemia [25-27], when hookworms are concerned. Furthermore, authors reported the existence of the increase of the risk for severe malaria occurrence among co-infected children by P. falciparum and helminth [28,29], which is not the case of our study which focused only on uncomplicated malaria. These results could be explained by the fact that non - specific type E immunoglobulin linked to the infection by A. lumbricoides for example seem to reduce the pathogenic effect of the specific type E immunoglobulin by saturating the type E immunoglobulin receptors [30]. However, a protecting role could come from the helminths notably A. lumbricoides during malaria. Thus, the asymptomatic forms of malaria should be studied because they can be likely to be associated to intestinal helminth infections [31]. In addition to single parasite infections, this study also demonstrated that no interaction exist between co-infections are very common in the study. Majority of children who were infected with P. falciparum were concurrently infected with one helminth species.
However, this association was not confirmed by multivariate logistic regression analysis and hence needs further investigations, similar to a study in Tanzania [32]. In contrast, previous studies observed a positive association between malaria and hookworm infection [33,34], but this parasite was not found in the current study.
The limits of this study were the exclusion of the probable impact of protozoa on this association and confounding factors like haemoglobin S, anaemia or other intrinsic host factors that make individuals less susceptible to malaria infection.
To conclude, this study shows a low prevalent of co-infection malaria and Soil-Transmitted helminths and a trend of a higher P. falciparum parasitic density among children infected by helminth mostly by A. lumbricoides. Despite the observation of contradictory results, it is important to note that co-infection malaria and intestinal helminths seem to have negative consequences on the clinical expression of malaria among children. The associations between malaria and helminth infections detected in this study were not conclusive and hence needs further investigation.
No conflict of interests was reported by the authors of this manuscript.
Kpongbo Etienne Angora, Vincent Djohan and Abibatou Konaté are the principal investigators of the study. Pulcherie Chistiane Kiki-Barro, Valérie Bedia-Tanoh, Kondo Fulgence Kassi, Henreiette Vanga-Bosson and Sébastien Miezan helped to the redaction of manuscript. Eby Hervé Menan, WillamYavo supervised the study. All authors contributed to the drafting of the paper.
We would gratefully thank the authorities and the personnel of the Anonkoua-Kouté urban and community health Center and the El-Rapha health center where this study was carried out as well as the patients and their parents for their participation. We also express our gratitude to the personnel of the Malaria Research and Control Center of National Institute of Public Health for its technical support.