A 41-year-old female patient received tigecycline because of abdominal infection caused by carbapenem-resistant klebsiella pneumoniae (CRKP) after performing cholangiolithotomy by the endoscopic retrograde cholangio-pancreatography (ERCP). Subsequently she developed cholestatic liver injury as substantiated by increased alkaline phosphatase (ALP) and bilirubin. Tigecycline was switched to dual carbapenems. Her liver function tests improved. We suggest that bilirubin levels should be cared in patients with tigecycline in order to avoid severe cholestatic liver injury.
Tigecycline, Cholestatic liver injury, Bilirubin
Tigecycline, a glycylcycline-class antibacterial, was developed in 2005 in the U.S.A., it has an expanded broad-spectrum antibacterial activity including multi-drug resistant pathogens. Its indications include complicated skin and skin structure infections, complicated intra-abdominal infections and community-acquired bacterial pneumonia [1]. Some adverse events of tigecycline were reported. The most frequent are nausea, vomiting, diarrhea, abdominal pain, headache, and increased alanine aminotransferase. Other, less frequent, adverse events such as increased alkaline phosphatase, total bilirubin concentration, prothrombin time, and pancreatitis were reported [2]. In this paper we present a case of cholestatic liver injury induced by tigecycline.
A 41-year-old female patient was admitted to hospital due to bile duct stones for two months after cholecystectomy. After performing cholangiolithotomy by the endoscopic retrograde cholangio-pancreatography (ERCP), the patient complained of abdominal pain. The white blood cell count (WBC) and procalcitonin (PCT) were elevated to 11.04 × 109/L and 153.44 μg/L respectively, while hepatic function was normal. The patient received tigecycline at the dose of 100 mg every 12 h and meropenem 1 g every 8 h because of abdominal infection caused by carbapenem-resistant klebsiella pneumoniae (CRKP, which was sensitive to tigecycline and resistant to other antimicrobial agents including carbapenems). After 8 days of tigecycline and meropenem intake, inflammation markers improved. However, her total bilirubin (TBIL), direct bilirubin (DBIL) and indirect bilirubin (IBIL) respectively elevated to 57.4, 33.94, 23.46 μmol/L. Tigecycline was reduced to 50 mg every 12 h. The levels of bilirubin continually increased, and then gradually decreased (Shown in Figure 1). The alkaline phosphatase (ALP) was two times higher than the normal, while alanine transaminase (ALT) and the aspartate aminotransferase (AST) returned to normal after a transient elevation (Shown in Figure 2). After 8 days of tigecycline 50 mg, the combination of tigecycline and meropenem was switched to dual carbapenems (meropenem 1 g every 8 h and ertapenem 1 g every 24 h) for CRKP. She was given ademetionine 500 mg every 12 h and acetylcysteine 4 g every 12 h, then her liver function tests gradually improved. Meanwhile her inflammation markers improved, the WBC and PCT were reduced to 7.02 × 109/L and 0.15 μg/L respectively.
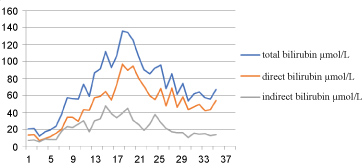 Figure 1: Dynamic changes in serum bilirubin levels over time.
View Figure 1
Figure 1: Dynamic changes in serum bilirubin levels over time.
View Figure 1
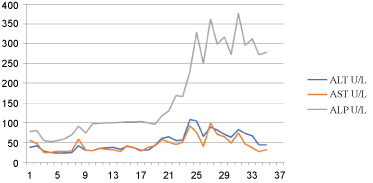 Figure 2: Dynamic changes in serum liver enzymes levels over time.
View Figure 2
Figure 2: Dynamic changes in serum liver enzymes levels over time.
View Figure 2
DILI is defined as serum alanine transaminase ≥ 5 times elevation of upper limit of normal (ULN) or alkaline phosphatase elevation ≥ two times ULN or alanine transaminase ≥ 3 ULN with elevation of bilirubin to ≥ 2 ULN. Hepatitic or cholestatic types of liver injury are defined by using R value (R = ALT ULN/ALP ULN); R ≥ 5 is suggestive of hepatocellular injury while R < 2 is suggestive of cholestatic injury and R value in between 2 and 5 is suggestive of mixed type of injury.
In this case, the TBIL, DBIL and IBIL increased obviously (the peak values were 136.09, 97.15 and 47.49 μmol/L) after starting tigecycline therapy. Peak ALP elevation (377 U/L) occurred on day 25 and exceeded the upper limit of normal for 2 times. Other liver function tests (ALT, AST) were normal or only mild elevated during tigecycline therapy. On admission, the patient denied any alcohol intake, smoking, the history of hepatitis, and her hepatic function was normal. After receiving antibiotic therapy, she was better except developed liver dysfunction. Therefore, the diagnosis of cholestatic liver injury induced by tigecycline was made (RUCAM score 6, probable).
Bonilla reported the impact of tigecycline on liver function mainly expressed as the increase in levels of liver enzymes (ALT, AST, GGT). In one single retrospective cohort (n = 528), 2% of the population who received tigecycline due to infection with multidrug-resistant (MDR) had an isolated elevation of ALP (peak ranging from 779 to 1869 U/L) with no other identifiable causes [3]. It is noted that cholestasis and jaundice were found to be relevant to tigecycline but with relatively low rates [4].
The main mechanism by which tigecycline provokes cholestatic liver injury is ambiguous. Tigecycline will increase hepatic fatty acid uptake and esterification in mice and induce steatosis [5]. Pessayre also reported tetracycline and the various tetracycline derivatives could lead to extensive microvesicular steatosis of the liver by inhibiting mitochondrial respiration and β-oxidation [6]. The side effects were observed more frequently in the high-dose group than in the approved dose group [4,7], though numerically higher efficacy values were observed with the tigecycline 100 mg twice-daily dose relative to lower doses of tigecycline combined with imipenem/cilastatin in the treatment of HAP [8].
In the research of [14C] tigecycline [9], it is concluded that the pharmacokinetics of tigecycline in serum showed a long half-life (55.8 h) and a large volume of distribution (21.0 L/kg). Excretion of unchanged tigecycline through feces was the primary route of elimination, which could be related with biliary passage function. It was demonstrated that compounds with biliary excretion significantly increased the incidence of jaundice (74% vs. 40%; P < 0.0001) [10]. Tigecycline accumulated in vivo after high dose application on a slow metabolism, and then it may activate immune-mediated bile duct injury with hyperbilirubinemia and markedly increased ALP.
For tigecycline there is no dosage adjustment required in patients with mild to moderate hepatic impairment, however in the patients with severe hepatic impairment (Child-Pugh C) the dosage of tigecycline should be altered to 25 mg every 12 h. Once drug induced cholestasis is suspected in a patient, discontinuation of the drug implicated is usually the first step. At the same time, it is also of major importance to assess the severity of cholestatic liver injury. Ursodeoxycholic acid (UDCA) and S-adenosyl-L-methionine are widely used for cholestatic liver injury.
Tigecycline induced cholestatic liver injury could be with lower incidence than that the increase of liver enzymes. The mechanism could be relative to the pharmacokinetics and esterification of tigecycline. High-dose tigecycline treatment is one of factors leading to the occurrence of adverse events. Consequently, we suggest that indicators, both liver function and bilirubin, should be closely monitored in patients receiving tigecycline, especially if high-doses and prolonged therapy is given.