Hepatitis B virus (HBV) is a virus that may lead to chronic infection of the liver. Once infected, HBV may stay in the blood and liver for a lifetime. HBV is a non-retro virus that infects hepatocytes, with an underlying mechanism of converting its relaxed circular DNA (rcDNA), into closed circular DNA (cccDNA). The cccDNA then serves as a transcription template to transcribe viral RNAs utilizing the transcription factors meant for the host system's transcription. At the moment, no RNA-based therapy has been approved by the FDA for HBV. An RNA interference-based silencing mechanism can silence the genes needed for the production of HBV mRNA transcripts. Currently, FDA-approved drugs for HBV include interferons and nucleos(t)ide analogs only. VIR Biotechnology, an American commercial-stage immunology company recently released data from a Phase 1 clinical trial of administering VIR-2218 (which is a small interfering ribonucleic acid siRNA). Further, they have initiated another ongoing phase 1 clinical trial using a combination of VIR-2218 and VIR-3434. The results from this trial (NCT05484206) are to be announced after March 2024 [1]. These are directed to have antiviral activity against HBV. VIR-2218 indicated siRNA may be used as a treatment for HBV as it showed reductions in HBsAg which is a viral surface antigen for HBV. In this article, a review has been provided to pinpoint how siRNA can be used against HBV as a potential treatment against the treatments already present.
Hepatitis B virus, HBV, Small interfering RNA (siRNA), Virus, siRNA therapy/treatment
HBV- Hepatitis B Virus
HBV remains a matter of global concern. One out of three people is affected by the Hepatitis B virus, with 1.5 million people being affected each year around the world. HBV can be contracted by the transmission or contact of bodily fluids; either through the sharing of needles, from mother to child at birth, or sexual contact. It is most commonly spread through vaginal, anal, or oral sex with someone who has the virus [2]. 820,000 people die each year after the contraction of HBV and the liver cancer that may follow. HBV is known to be the number one reason for liver cancer; with liver cancer being the second most common cause of cancer-related deaths worldwide. In the United States, approximately 2.4 million people are victims to HBV related chronic infections. Furthermore, after the increased use of opioids in the United States, hepatitis B infection rates are known to have risen by 50%-450%. In actuality, only 25% of the individuals who have contracted the virus are diagnosed [2].
At the moment, no RNA based therapy has been approved by the FDA for HBV. Current FDA approved drugs for HBV include interferons and nucleos(t)ide reverse transcriptase inhibitors (NRTIs) only [3]. The downsides of these treatments will be discussed in the following section.
There are 7 main proteins that are produced by the HBV genome. These are hepatitis B Core antigen, pre-Core, Small S, Middle S, Large S, Polymerase, and critical HBV-encoded regulatory protein hepatitis B virus X protein (HBx) [4]. SiRNA therapy is capable of silencing the entire HBV genome which means these proteins would not be produced. It is a wholesome and sustainable way to combat HBV.
NTRIs target and inhibit the production of viral cccDNA from pregenomic RNA (pgRNA) (by reverse transcription). NTRIs need to be administered throughout a patient’s life as once the dosage is stopped, the cccDNA would continue to be produced as there is no inhibitor to stop reverse transcription of the pregenomic RNA (pgRNA) into the minus strand DNA that leads to plus strand DNA replication and consequently viral cccDNA production for HBV (Figure 1).
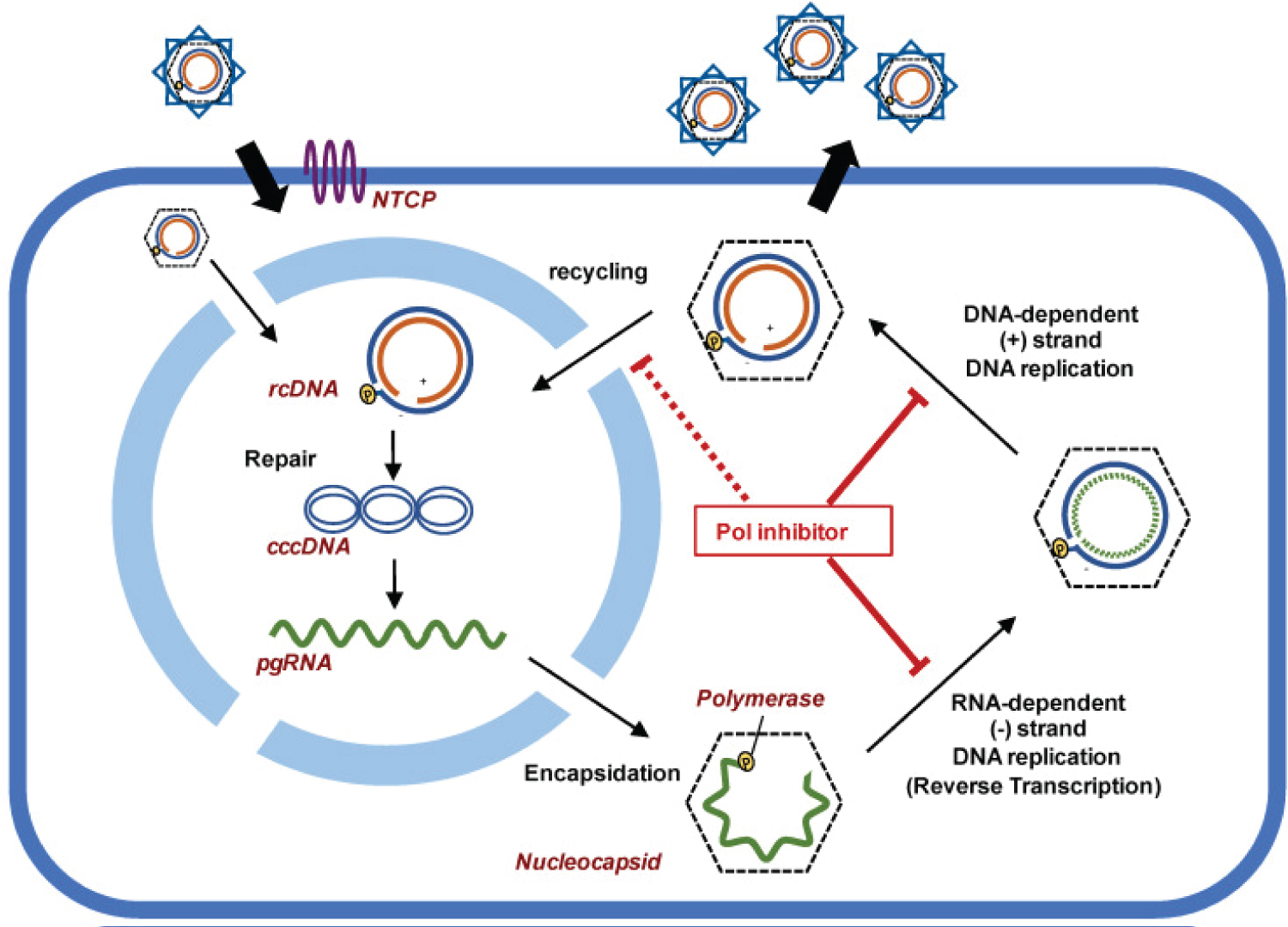 Figure 1: Working mechanism of NTRIs [5].
View Figure 1
Figure 1: Working mechanism of NTRIs [5].
View Figure 1
This would mean that upon retraction of the nucleos(t)ide reverse transcriptase inhibitors (NRTIs) medication, HBV symptoms and Hepatitis B surface antigen (HBsAg) would surface again.
Henceforth, a more promising and long term treatment can be considered for HBV. Results from phase 1 clinical trials by VIR biotechnology have shown a significant reduction in HBsAg levels in the blood. Vir biotechnology administered a dosage of VIR-2218 (which is a small interfering ribonucleic acid siRNA) in 49 participants. On December 13, 2021 , VIR Biotechnology declared that by giving participants VIR 2218 for 24 or 48 weeks alongside 48 weeks of pegylated interferon alpha (PEG-IFN-α) (cohorts 4 and 5 combined), 16% or 5/31 of the participants exhibited HBsAg loss 24 weeks after the end of treatment [6].
SiRNA is capable to regulating the expression of genes, and in this case VIR2218 can silence all HBV viral RNAs. Its efficiency lies in the ability to target the region of the HBV genome that is common to all viral transcripts. It can be synthesized in vitro . SiRNAs were discovered first in 1999 in plants at The Sainsbury Laboratory (TSL) in England and were made to guide sequence-dependent Endo nucleolytic cleavage of the mRNAs whereby the antisense RNA is complementary to the targeted mRNA [7].
The mechanism for the activity of siRNA is essentially by the digestion of double stranded RNA by DICER and is as follows; Dicer (endoribonuclease dicer) is a helicase enzyme that has an RNase motif. Dicer is encoded by the DICER1 gene. It gives rise to siRNA by cutting down long dsRNA that are present in the genome. Now, the siRNA has two nucleotide long, unpaired overhangs at the 3′-end of both strands. Upon formation, they now bind to a protein complex known as the RISC complex (RNA induced silencing complex). Among the siRNA strands, the strand with the more stable 5′-end is typically integrated to the active RISC complex. This is known as RISC activation. A catalytic RISC protein, a member of the argonaute family (Ago2), now helps the single stranded siRNA (the guide or antisense strand) and RISC complex to aim and orient towards the mRNA that is targeted for cleave and henceforth, degradation. The target mRNA is deciphered on a homology dependent basis with the guide RNA strand.
Once the viral mRNA transcripts are cleaved, they are no longer viable for the translation of the amino acids. This halts the translation process and proteins cannot be produced from the transcript. For HBV, VIR 2218 targets all HBV viral mRNAs, therefore underproducing all HBV viral proteins (Figure 2).
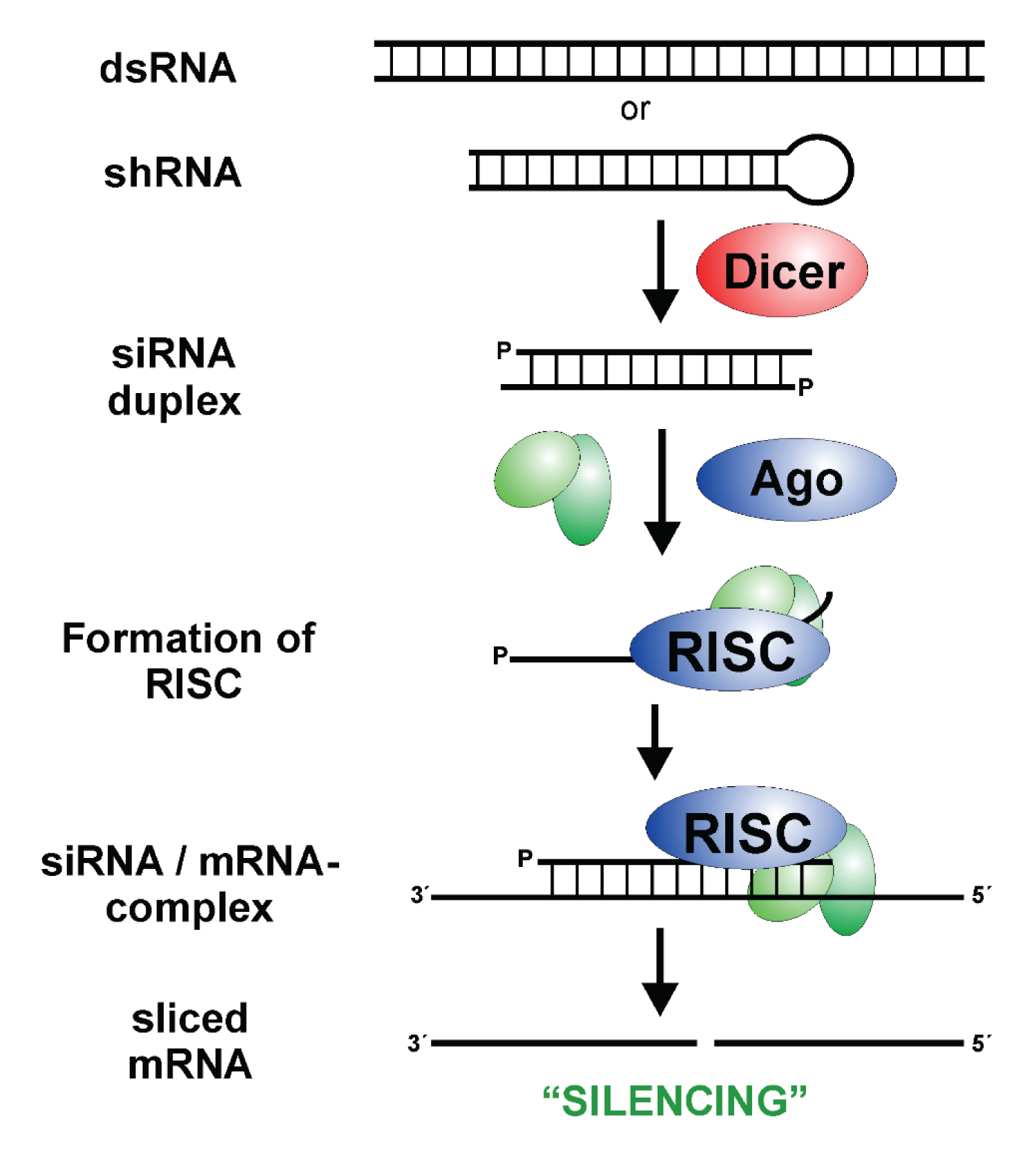 Figure 2: Small Interfering RNA; RISC: RNA Induced Silencing Complex [8].
View Figure 2
Figure 2: Small Interfering RNA; RISC: RNA Induced Silencing Complex [8].
View Figure 2
The preclinical study on rats after a single subcutaneous dose of 12 mg/kg, 36 mg/kg, or 100 mg/kg (n = 4 mice per dosing group) on Days 0, 21, 28, 35, and 42.
Liver, tissue and blood samples were collected. ALT levels were reportedly lower following VIR2218 administration. ALT refers to alanine aminotransferase and helps break down proteins in the liver. When the liver is damaged, ALT may be released into the bloodstream. High ALT blood levels indicate liver diseases [9] (Figure 3).
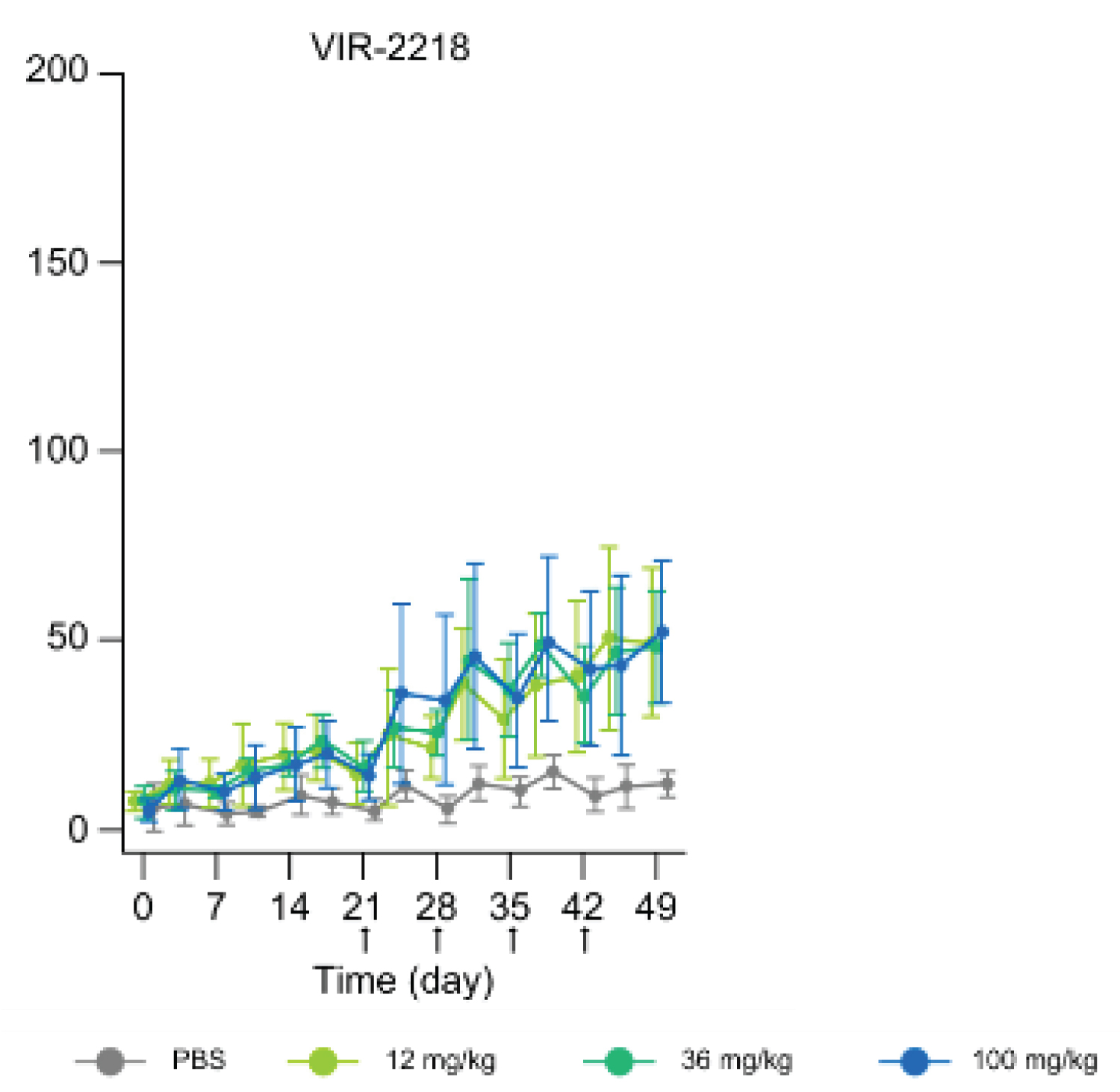 Figure 3: A graph showing ALT levels throughout the VIR 2218 treatment [10].
View Figure 3
Figure 3: A graph showing ALT levels throughout the VIR 2218 treatment [10].
View Figure 3
To test the safety and tolerability of a single dose of VIR-2218, a phase 1 clinical trial (NCT03672188) was carried out. It was a Phase 1/2, Randomized, and Placebo-Controlled Study. The placebo condition went up to a phase 2 clinical trial. The participants had to have a BMI of 18-32 kg/m^2 and ages 18-65 (male or female). Lastly, they had to have a Chronic HBV infection for >/= 6 months [11].
The study design was as follows in Figure 4.
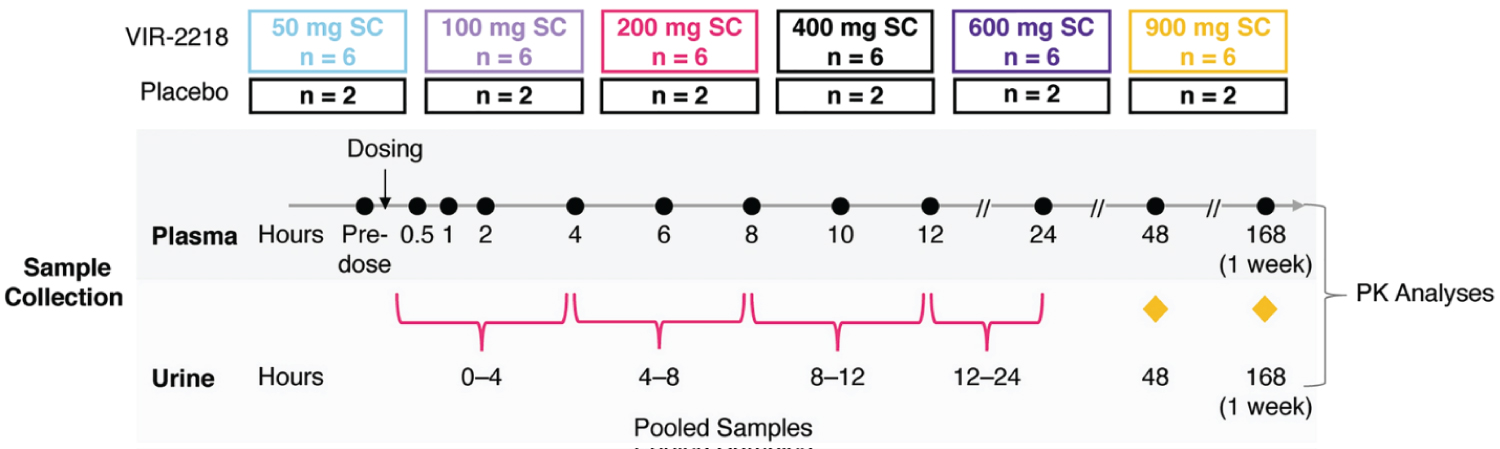 Figure 4: Study design and outline for VIR-2218, a phase 1 clinical trial [12].
View Figure 4
Figure 4: Study design and outline for VIR-2218, a phase 1 clinical trial [12].
View Figure 4
As for the pharmacokinetic behaviour seen for VIR 2218 as a result of this phase 1 clinical study (NCT03672188), the drug was instantly absorbed from the blood plasma and directed to circulation after being given subcutaneously. VIR-2218 is an ESC+ GalNAc–siRNA conjugate and with the help of GalNAc, the drug was up taken in the liver speedily [12].
According to VIR Biotechnology, the preliminary 48-week safety and efficacy data, VIR-2218 was administered in combination with PEG-IFN-α (a Pegylated interferon used as an immunoregulatory drug) during the phase 1 trial as a subcutaneous injection. The trials included placebo, double-blinded and randomized controls among healthy and (HBV) infected participants. There were 8 participants per cohort. 2 for placebo and 6 for the experimental group. They were either given the placebo drug or VIR 2218 in combination with PEG-IFN-α.
The longer the participants received the drug, the more reduction in the amount of HBs (surface antigen) was seen. The maximum time span allotted was 48 weeks. There was a mean decrease of -2.9 log10 IU/mL) in HBsAg when VIR-2218 was administered in combination with PEG-IFN-α. 10 participants receiving VIR-2218 with PEG-IFN-α gained HBsAg seroclearance (loss of circulating HBsAg) by week 48, and nine achieved anti-HBsAg levels which were > 10 mIU/mL.
Patisiran is an example of a siRNA based therapy that targets hereditary transthyretin amyloidosis (hATTR) by a lipid nanoparticle delivery system. It was approved in 2018 by the FDA following the phase 3 clinical trials NCT01960348 that ended in 2017. Patisiran cleaves and degrades all mRNA transcripts of transthyretin (TTR). This includes the mutant mRNA as well as the wild type. Consequently, it reduces the amount of TTR protein circulating, therefore improving neuropathy and cardiac function [13] (Figure 5).
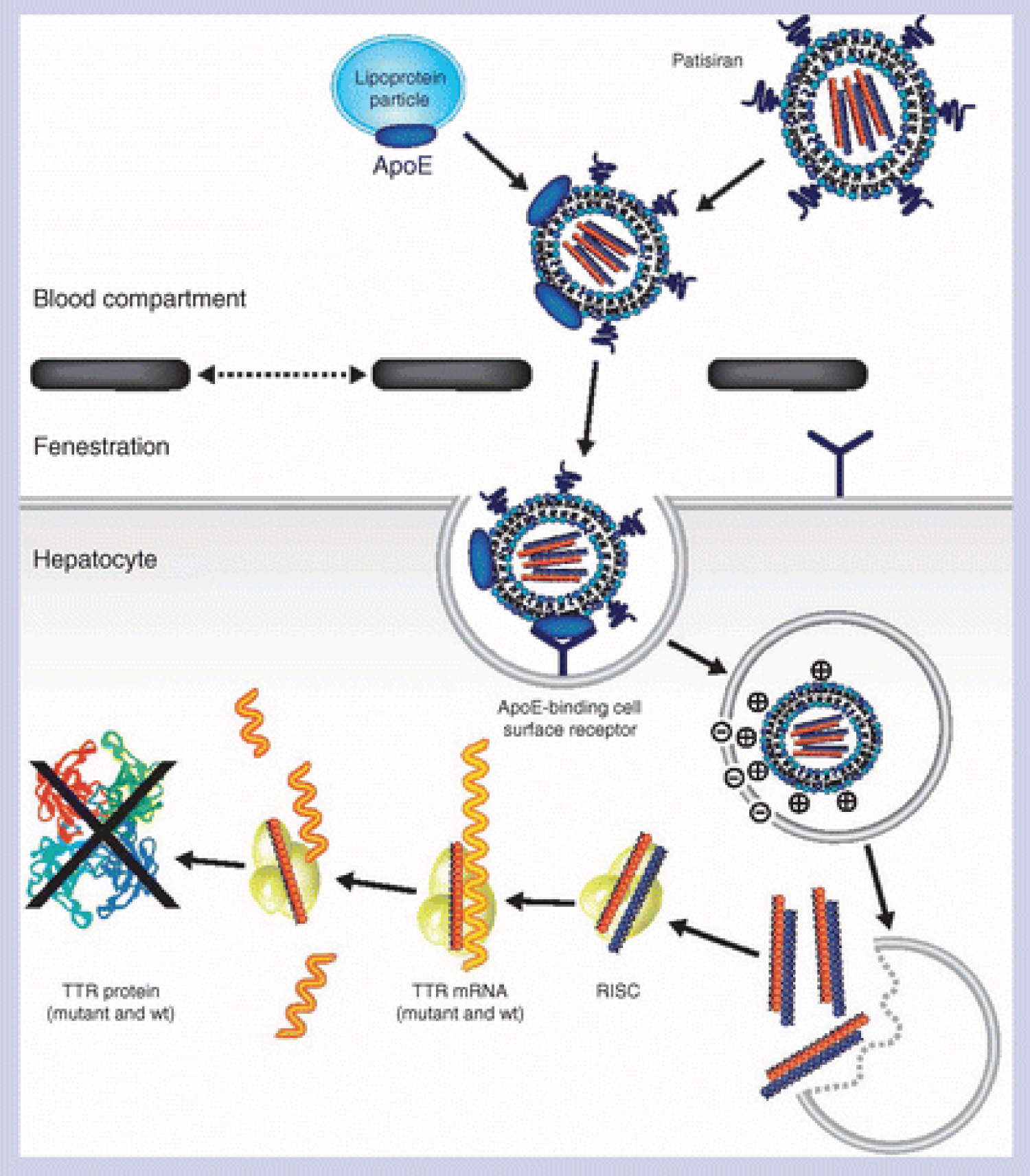 Figure 5: Working principal for Patisiran [14].
View Figure 5
Figure 5: Working principal for Patisiran [14].
View Figure 5
Another FDA approved drug that is siRNA based is Givosiran. Givosiran deals with degrading aminolevulinate synthase 1 (ALAS1) mRNA. It targets acute hepatic porphyria (AHP) which is a genetic disorder that affects the liver and the nervous system. According to the results of the registered clinical trial; numbered NCT03338816, Givosiran reduced the manifestation of the disease [15].
Moving on to another FDA approved siRNA drug, Lumasiran. It is sold under the brand name Oxlumo and treats primary hyperoxaluria type 1 (PH1). Mutations or defects in the alanine glyoxylate aminotransferase ( AGXT) gene in the liver disrupt the function of the enzyme glycolate oxidase which is specific to the liver. Glycolate oxidase thereby converts glyoxylate into oxalate which the human body can’t metabolize. Lumasiran is a synthetic double-stranded siRNA that is conjugated with the N-acetyl galactosamine (GalNAc) covalently [16]. Lumasiran inhibits the enzyme glycolate oxidase (GO) which is responsible for producing glyoxylate which is a precursor and further leads to the synthesis of oxalate which is toxic at high levels [13].
Lastly, another success story for siRNA is Inclisiran (brand name Leqvio). Inclisiran is also a liver-based siRNA that targets and degrades the hepatic mRNA that translates the proprotein convertase subtilisin/kexin type 9 (PCSK9). This protein is expressed in people with atherosclerotic cardiovascular disease, ASCVD. PCSK9 disrupts cholesterol receptors (LDL receptors) on liver cells that aim to remove LDL (low-density lipoprotein) cholesterol (otherwise known as bad cholesterol) from the blood. If the receptors on the cells are disrupted, LDL-c cannot move into the cells for lysosomal degradation. This leads to high levels of LDL- c in the blood and therefore high risk of ASCVD. Inclisrian removes this risk by degrading PCSK9 so the LDL receptors are not disturbed and the LDL-c can easily move into the liver cell for degradation [17].
In order to make VIR 2218 commonplace in the drug market, the results from the Phase 1 clinical trial (NCT05484206) should be evaluated. The official end date for the study is in March 2024. This new trial is currently evaluating the safety and pharmacokinetic behaviour for VIR 2218 in combination with VIR 3434.
It is imperative to note that according to Gupta, et al. [12], the pre-clinical studies suggested that after VIR 2218 dosage, the HBsAg and ALT levels declined; however, the HBsAg levels continued to decline even after VIR-2218 remained undetectable in plasma. Therefore, the amount of dosage and the HBsAg level decline after absorption from plasma into the liver (when all the drug has disappeared from the plasma) should be considered in future clinical studies using a pharmacokinetic/pharmacodynamics PK/PD model. That being said, pre-clinical studies indeed establish a correlation between total siRNA levels in the liver delivered using a GalNAc–siRNA conjugate and the silencing activity of mRNA transcripts. GalNAc is a synthetic triantennary N -acetyl galactosamine ligand that enables efficient delivery of the siRNA to the liver cells [18].
Various studies have shown immediate target suppression using RNA therapy, such as, cemdisiran, for the treatment of complement-mediated diseases; vutrisiran reduced transthyretin production, etc. The prolonged prevalence of VIR 2218 in the liver cells is anticipated to target the HBV viral genome [12].
Furthermore, GalNAc-siRNA conjugates delivery system allows enhanced stability of the siRNA at the 5 prime end, improving its efficacy and silencing abilities [18].
Since HBV affects 1.5 million people per year worldwide, it is a massive concern. Conclusively, due to the mechanism of silencing action of VIR 2218, it is a novel and potential treatment for Hepatitis B virus that shows plausibility and promise. With the advent of VIR 2218, and with the further ongoing studies using VIR 2218 in conjugation with VIR 3434, much promise can be shown for anti-viral therapies using siRNA. VIR 2218 is able to target and inhibit HBV replication and the delivery system using GalNAc-siRNA conjugates is also precise as prior studies have suggested. Phase 1 clinical trials from dosages of VIR 2218 suggested that the drug uptake into hepatocytes was rapid and there were no safety concerns. In the years to come, a phase 2 clinical trial can be initiated to further gather data on efficacy. Moreover, the VIR 2218 and VIR 3434 phase 1 clinical study results can be evaluated (once announced in March 2024) to see the safety and effect of two siRNAs in conjugation. While challenges remain, the results presented in this article suggest that VIR-2218 may be an essential treatment that may lead to an HBV-free future. It is also imperative to consider whether a booster dose may be required after the VIR 2218 dose.
Compared to other therapies that exist currently for HBV, interferons, and nucleos(t)ide reverse transcriptase inhibitors, siRNA mediated therapy has a higher success rate due to higher specificity as it has a high complementarity to the HBV transcripts. NTRIs need to be administered throughout a patient’s lifetime whereas siRNA mediated treatment would destroy all HBV transcripts. The effects of siRNA are very long-lasting and the patient does not need to receive the drug frequently. However, the possibility of off-target cleavage should not be ignored and should be tested for in the upcoming trials.
Lastly, as siRNA therapy is not commonplace, convincing publications of the positive results from trials of different siRNA success stories (as listed above) should be circulated to inform people of how effective siRNA therapy is compared to more traditional forms of therapies. Having compared and contrasted other available FDA options in this review, the benefits of siRNA therapy using clinical trial results of HBV, while also mentioning previous success rates of siRNA therapies, siRNA is indeed a viable option for treatments and should be more commonplace in the future as trials progress (especially for HBV). As HBV is a very common infection that is easily transfected, it is essential to highlight and review any new and upcoming treatments for it.
The author has received no financial or academic support for the research, authorship, and publication of this article.
None to declare.
This author takes responsibility for all aspects of the reliability and freedom from bias of the data presented and their discussed interpretation.