Active and chronic antibody-mediated rejection (AMR) is a common cause of graft failure. Prognostic markers of this complication are not well defined. We aimed to find out the demographic, histopathological and clinical characteristics of transplant recipients who developed AMR and to evaluate the impact of these features as well as antirejection treatment modalities on graft survival.
Thirty -two patients who developed AMR (22 male; mean age 40.59 ± 12.52 years) were included in the study. Data were investigated retrospectively and graft survival was analyzed. All transplant biopsies were evaluated according to Banff 2013 classification.
Of the 32 cases, 26 were transplanted from living-donors. Mean serum creatinine at time of biopsy was 1.99 ± 0.09 mg/dL. Proteinuria was 1566.06 ± 353.92 g/day at the time of biopsy. The need for hemodialysis was significantly related with initial creatinine (p = 0.003); creatinine after three months (p < 0.001) and final creatinine (p < 0.001) as well as initial proteinuria (p = 0.005); proteinuria after three months (p < 0.001) and final proteinuria(p < 0.001).6 cases showed diffuse C4d positivity, 26 cases showed focal c4d positivity. Five of 6 patients with diffuse C4d staining in renal biopsy were hemodialyzed at first and third months despite antirejection therapy(p = 0.029 and 0.041, respectively). Mean survival time was 1654.67 ± 220.40 (95% CI = 1222.68-2086.66) days for focal staining C4d cases and 366.16 ± 36.44 (95% CI = 294.73-437.60) days for diffuse staining C4d cases. The difference was statistically significant (p = 0.012). Two of the patients died, 15 experienced graft loss and 17 survived with functioning grafts. Mean survival time between antirejection treatment modalities showed no statistical significance (p = 0.15).
Serum creatinine, proteinuria at time of biopsy, diffuse peritubular C4d stainig were significantly associated with graft survival. Early diagnosis is important to improve success in the treatment of AMR and graft survival
Renal transplantation, Antibody-mediated rejection, Graft survival
Active and chronic antibody-mediated rejection (AMR) is a common cause of graft failure. In previous reports the incidence of AMR has been defined to vary between 5.6% and 23%. AMR frequently occurs as a reaction to donor HLA antigens and seldom to non-HLA antigens [1,2]. ABMR's revised Banff 2017 classification, active and chronic active AMR, histological evidence of acute and chronic damage, recent antibody interaction with the vascular endothelium, and conditions associated with donor-specific antibodies (DSA) to human leukocyte (DSA) definitions as. HLA) or non-HLA antigens [3]. B cell and plasma cell activation leads to the production of DSAs that bind to HLA or non-HLA molecules expressed on endothelial cells in the renal allograft [4]. Active AMR contributes to peritubular capillaritis, glomerulitis, and rapid decline in allograft function [4]. Chronic AMR manifests histologically as transplant glomerulopathy and result in progressive decline in kidney function [5]. The diagnosis of AMR is based on three key criteria: (1) histological evidence of acute-chronic tissue damage, [2] evidence of antibody interaction with vascular endothelium (peritubular capillary C4d accumulation), and [3] determination of circulating DSAs [6]. Multiple studies have shown an association between peritubular c4d staining, DSA and pathological findings in patients with AMR [7].
AMR treatment after diagnosis is based on 2 basic mechanisms; (1) Suppression of B cells or plasma cells, (2) removal of donor specific antibodies. In the current study, we aimed to find out the demographic, histopathological and clinical characteristics of transplant recipients who developed AMR and to evaluate the impact of these features as well as antirejection treatment modalities on graft survival.
The data from 306 kidney transplantations performed between January 2012 and June 2018 were retrospectively analysed; 32 recipients has been found to develop AMR. The data from files of these patients were evaluated and demographic, histopathological and clinical characteristics were recorded. Demographic data including age and gender; clinic5al data including the underlying etiology for chronic kidney disease, duration of dialysis, renal replacement therapy; laboratory data including serum creatinine, urinary protein, immunological characteristics and histopathological features of rejected kidney were recorded. The biopsies from transplanted organ were evaluated according to Banff 2013 classification. PRA class 1 and 2 were studied during diagnosis and at 3 months after treatment; DSA detection could not be performed due to financial problems. According to the protocol of our transplant center, all patients received induction therapy with ATG.
As a standard immunosuppression regimen, 30 of the 32 patients were taking tacrolimus + mycofenalic acid derivative + prednisolone, two patients receiving tacrolimus+mycofenalic acid derivative. Treatments given after diagnosis of AMR were analyzed retrospectively. The study is approved by the local ethics committee.
Quantitative data were expressed as mean values and standard deviations (mean ± SD). Graft survival was evaluated by using Kaplan-Meier method, and Cox regression analysis was used for finding out independent factors. Chi-square and T-tests were used for comparison of two groups. A p value of less than 0.05 was considered statistically significant. Statistical analysis was performed by Statistical Package for Social Sciences for Windows version 17.0 (SPSS Inc, Chicago, IL) software.
Of 306 kidney transplantations performed in our centre, 32 recipients has been found to develop AMR (10.4 %). The study population consisted of 22 male and 10 female patients. The mean duration of RRT was 27.22 ± 18.62 months. The mean age at the time of diagnosis was 40.59 ± 12.52 (20-63) years. The majority of the patients(n = 26, 81.3 %) have been transplanted from living donors. Five female patients' past medical history were characterized by pregnancies, while 12 patients received blood transfusion. The patients' demographic characteristics are shown in Table 1.
Table 1: The patients' demographic characteristics. View Table 1
Mean serum creatinine level was 1.99 ± 0.09 (1.36-3.70) mg/dL. Proteinuria was 1566.06 ± 353.92 g/day at the time of biopsy. Laboratory and immunological parameters of the patients at the time of biopsy are given in Table 2. Pre-transplant and at the moment of biopsy immunological characteristics are shown in Table 3. AMR was diagnosed at a mean of 38.34 ± 29.23 (6-120) months post-transplantation. Half of the cases were diagnosed as active AMR (n = 16); 7 of these cases were started hemodialysis at first month( 43.8 % of acute AMR cases). Of 16 chronic AMR cases, 6 cases( 37.5 %) underwent hemodialysis at the first month after treatment. The difference between theses two groups was not statistically significant (p = 0.71). In the third month, 7 of 16 active AMR ( 43.8 %) and 8 of chronic AMR cases (50.0 %) underwent hemodialysis. The difference between these two groups was not statistically significant (p = 0.72).
Table 2: Laboratory and immunological parameters of the patients at the time of biopsy. View Table 2
Table 3: Pre-transplant and at the moment of biopsy immunological characteristics. View Table 3
The histopathology of the allograft biopsy specimens with AMR is shown in Table 4. C4d deposition in PTC was observed in all cases as either diffuse (6) or focal (26) staining No significant difference was determined in terms of graft survival when we divided our patients into two groups based on microvascular inflammation (MVI) score (patients with MVI score ≤ 3 and > 3) (p = 0.086). When the group with transplant glomerulopahty (TG) is compared to the non-TG group, the frequency of dialysis need despite treatment at the third month was not statistically significant P = 0.83).
Table 4: The histopathology of the allograft biopsy specimens with AMR. View Table 4
Table 5: Anti-rejection treatments and outcomes received by AMR patients. View Table 5
Anti-rejection treatments and outcomes received by AMR patients. Tablo5 As rejection therapy, the patients were determined to have received one or more of pulse steroid , intravenous immunoglobulin (IVIG), rituximab, plasmapheresis. After initiation of anti-rejection therapy, 13 (40,6%) patients were administered hemodialysis at first month and 15 (46.9) patients at the third month. Two patients (6.3%) were deceased. Thirteen patients (61.9%) who were positive for PRA class 1 at the time of renal biopsy returned to hemodialysis despite antirejection treatment after one month(p < 0.001). All 15 cases who underwent hemodialysis at the third month were positive for PRA class 1 (p < 0.001) and PRA class 2. Five of 6 patients with diffuse C4d staining in renal biopsy were hemodialyzed at first and third months despite rejection therapy (p = 0.029 and 0.041, respectively). When we compared the groups with and without TG, the response to antirejection treatment was not significantly different (p = 0.68). The need for hemodialysis was significantly related with initial creatinine (p = 0.003); creatinine after three months (p < 0.001) and final creatinine (p < 0.001) as well as initial proteinuria (p = 0.005); proteinuria after three months (p < 0.001) and final proteinuria(p < 0.001).
Kaplan–Meier survival analyses based on antirejection treatment modalities and C4d staining are demonstrated in Figure 1, Figure 2, respectively. Mean survival time was 1654.67 ± 220.40 (95% CI = 1222.68-2086.66) days for focal staining C4d cases and 366.16 ± 36.44 (95% CI = 294.73 - 437.60) days for diffuse staining C4d cases. The difference was statistically significant (p = 0.012). Mean survival time concerning antirejection treatment modalities were as follows: 827.25 ± 294.59 (95% CI = 249.84-1404.65) days for those who received corticosteroids, plasmapheresis, IVIG; 949.22 ± 234.80 (95% CI = 489.00-1409.44) days for those who corticosteroids, plasmapheresis, IVIG and rituximab and 1883.46 ± 280.15 (95% CI = 1068.49-1861.51) days for those who received corticosteroids, IVIG and rituximab. The difference between these three groups showed no statistical significance (p = 0.15). By multivariate analyses, none of the covariates was found to have an independent impact on graft survival.
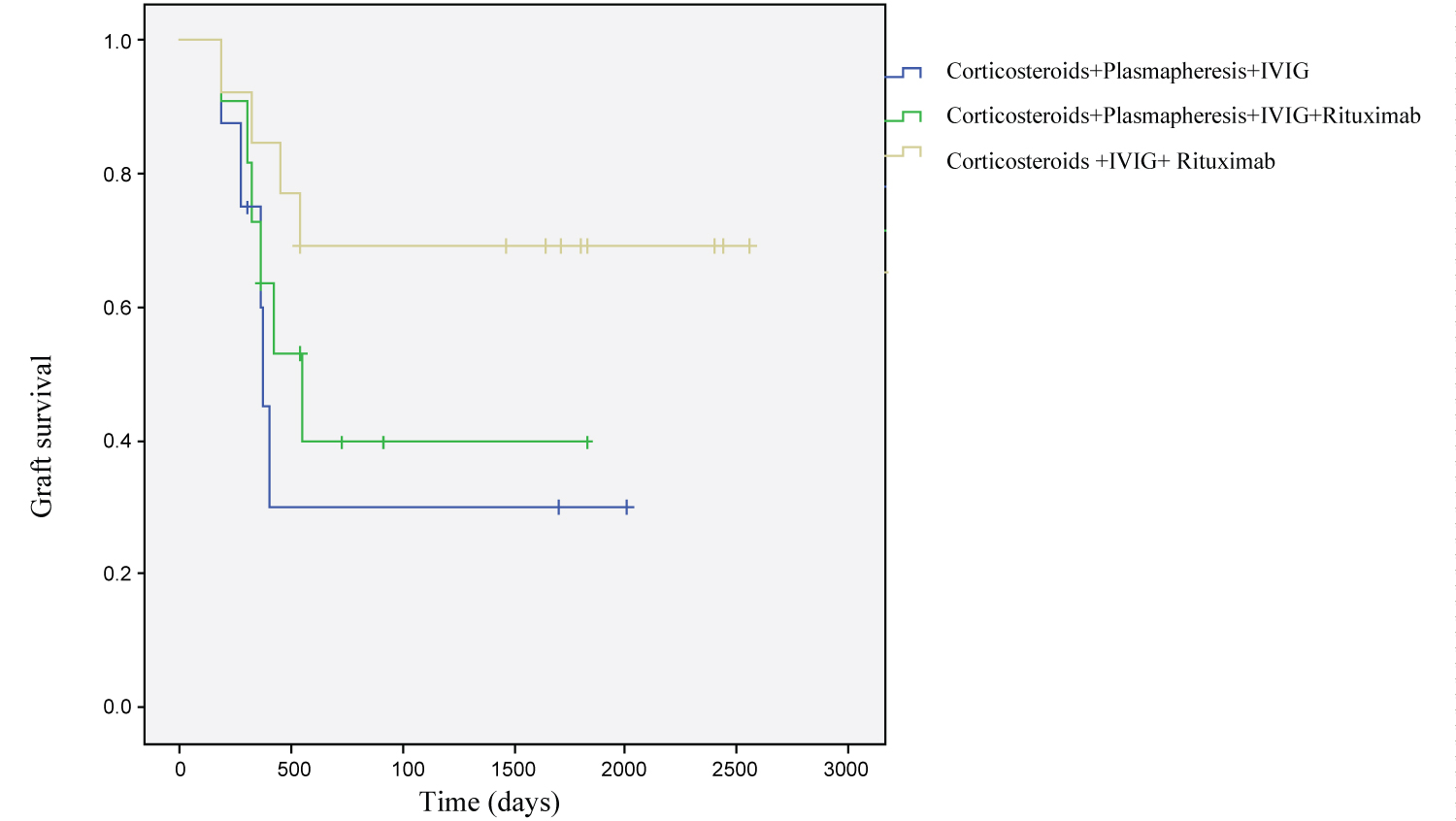 Figure 1: Survival functions according to anti-rejection treatment.
View Figure 1
Figure 1: Survival functions according to anti-rejection treatment.
View Figure 1
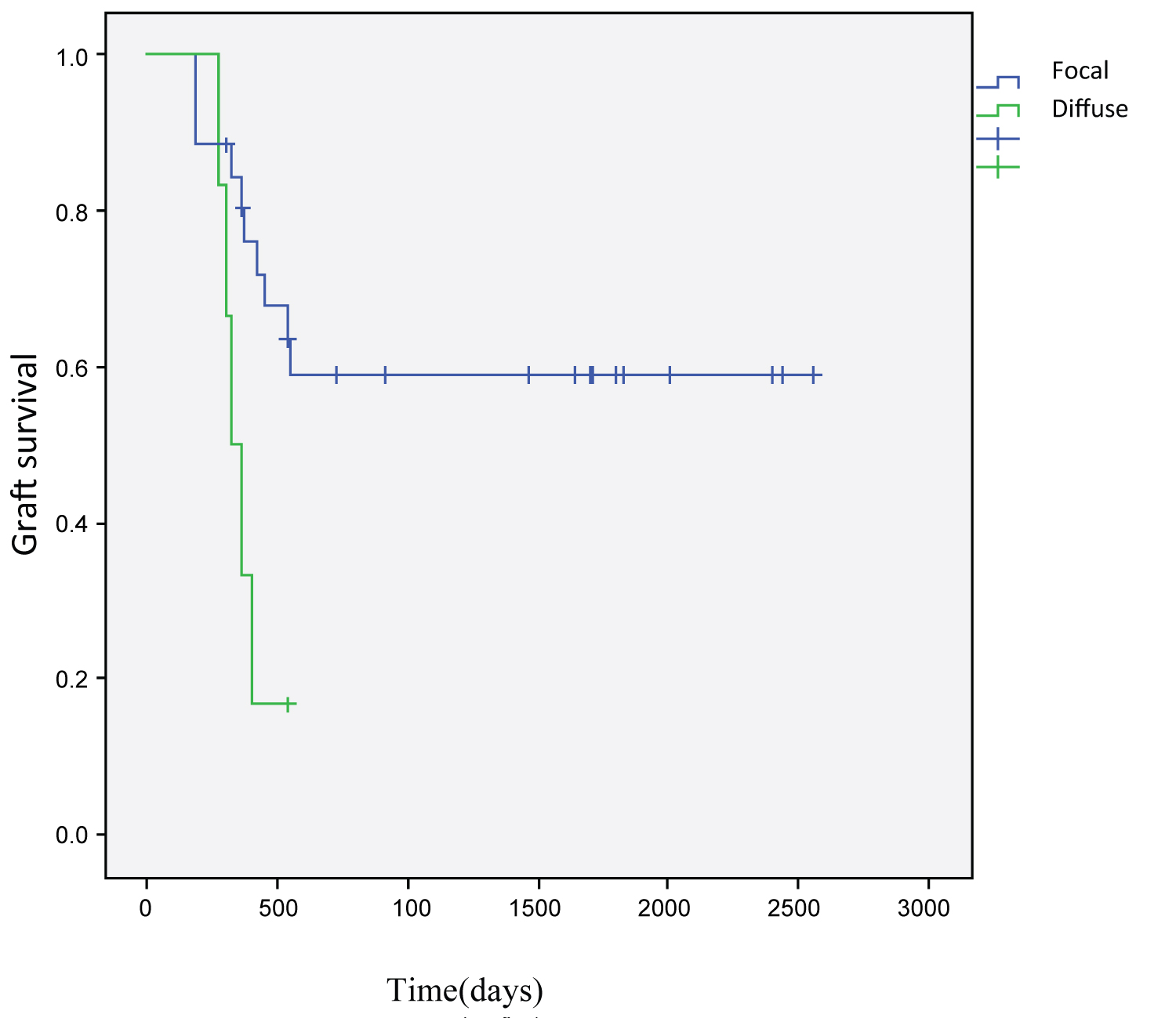 Figure 2: Survival functions according to C4d staining.
View Figure 2
Figure 2: Survival functions according to C4d staining.
View Figure 2
Active and chronic antibody-mediated rejections are dangerous entities with significant effects on patient and graft survival. In our study, a statistically significant relationship was detected between creatinine, proteinuria, PRA percentage at the beginning of rejection and diffuse C4d staining in renal biopsy and graft survival. In our patients, the mean serum creatinine levels at time of biopsy was 1.99 mg/dl. In previous studies, there are data on mean serum creatinine levels of 1.6-1.7 mg/dl [8,9]. Akagün, et al. studied on 52 AMR patients and found serum creatinine ratio to be 3,8 mg/dl at the time of biopsy. An important finding in our study is a negative correlation between mean serum creatinine levels at the time of biopsy and graft survival. Akagün et al showed that serum creatinine levels at time of biopsy were correlated with graft survival (p = 021) [10] . Mean serum creatinine values were 2.49 ± 0.24 mg/dl in the third month of treatment. In the study conducted by Yılmaz et al, the mean blood creatinine value was 3.1 ± 1.4 at the last follow-up visit and mean proteinuria was 2300 (1300-3300) mg/day [11]. These results were in line with our results. Mean time to diagnosis of rejection was 38.34 ± 29,.23 months in our cases; in other words, it can be considered as late acute rejection. Consistent with our study, Akagün et al found the posttransplant AMR diagnosis time as 34.5 months [10]. Yılmaz et al showed that mean duration between transplantation and the development of AMR was 31.9 ± 25.9 months [11]. A significant difference was not found in terms of graft survival between cases in which rejection occurred in the acute period and those in which it occurred in the chronic period (p = 0.72). We have demonstrated that graft survival was significantly shorter in PRA positive cases at biopsy time. This finding is consistent with another study which showed that graft function and high PRA levels during biopsy were predictors of graft loss [12]. The mean creatinine was 1.93 mg/dl in the group with focal C4d staining, whereas it was 2.23 mg/dl in the group with diffuse C4d staining.
Several studies on the association between graft survival and C4d staining have reported different results. Diffuse positivity of C4d in peritubular capillaries is known to be a poor prognostic factor [13]. We have demonstrated shorter graft survival in C4d positive cases, consistent with previous studies [13,14]. When we divided our patients into two groups based on MVI (glomerulitis+peritubular capillaritis) score (MVI score_3 and > 3), one recent mode of assessment in biopsies of renal transplant patients [15], we have found no significant difference in terms of graft survival. Our results may not have reached statistical significance due to our low case numbers.In a study involving 8 patients Faguer et al have reported a 75% graft survival rate over a 10-month follow-up period following plasmapheresis and rituximab treatment [8]. In another study, graft survival rate in 569 days following administration of plasmapheresis and IVlG has been reported to be 81% [16].
In our study, there was no relationship between treatment modalities and graft survival. In previous researches, IVIG therapy has generally been administered in combination with plasmapheresis and rituximab [8,9]. Plasmapheresis was administered to 19 of 32 patients in our research, and IVIG to 32. However, in our study, there was no statistical significance among IVIG-containing treatment groups. Consistent with these results, treatment modalities specific to antibody mediated rejection must be administered. The loss of graft due to AMR was found to be 15.3% in a study conducted by Yalçıntaş, et al. [17]. In another study, seven (50%) patients lost their grafts [11]. This ratio was 46.9% in our study. This may be due to the patient's late referral to the transplant unit for allograft biopsy, and may also be a feature of the destructive process of AMR.
In a study published in 2007, the estimated patient survival was 99% and the graft survival was 80% over an average follow-up period of 18 months [18]. In our study, despite the treatment, the loss of grafts was 46.9% in the mean follow-up period of 31 months. In our study, two patients (6.3%) died. Patient survival rate was 100% in the study conducted by Yılmaz, et al. [11]. In our study, the patient survival rate was 93.75%. There are several limitations of our study. First, our study is retrospective. The second is that our study did not contain as many cases as to make clearer statistical comments. The third is that DSA cannot be studied due to financial problems.
In conclusion, AMR has a significant and negative effect on graft survival. In our study, serum creatinine level, proteinuria at the time of biopsy, C4d staining adversely affected graft survival. Early diagnosis is important to improve success in the treatment of AMR and graft survival. As a result, regular creatinine, proteinuria, PRA follow-up and rapid renal biopsy is recommended in case of possible rejection.