Improvement in short term outcomes after kidney transplant has been achieved by using different induction and maintenance therapeutic approaches. Long term outcomes have not matched the expectations of the transplant stakeholders. Our study aimed to address the early impact of induction agents on long term outcome of kidney transplant when measured by Iothalamate clearance.
All adult kidney transplant recipients between January of 2012 and December of 2016 were reviewed. 649 patients were divided into three groups based on the induction agent (Basiliximab, Alemtuzumab, and Thymoglobulin). Protocoled 4 months and 48 months kidney allograft function evaluation with Iothalamate clearance test were compared among the three groups.
Patients who received Basiliximab were significantly older with no difference among African American and Caucasians. The 48 months assessment showed significant decline in median Iothalamate clearance in Basiliximab group at 49 ml/min vs. Alemtuzumab group 64.8 ml/min and Thymoglobulin 60 ml/min with p = 0.007. The Basiliximab group developed a significant higher proteinuria measured by spot urine to creatinine ratio at 48 months.
The use of Basiliximab as an induction agent for kidney transplant is associated with significant decline in kidney function 5 years post-transplantation when measured by Iothalamate clearance.
Induction therapy, Iothalamate clearance, Kidney allograft
With development of immunosuppressive medications and major advances in clinical care, short-term outcomes after kidney transplant are noticeably better [1,2]. However, the main challenge is how to improve long-term outcomes [3,4]. The introduction of potent and selective agents for the initiation of immunosuppression, as induction therapy, has reduced the incidence of acute rejection early post-transplantation and improved one-year graft survival [5-11]. However, long term graft survival was less linked to induction therapy and more to maintenance immunosuppressive regimen and existence of medical comorbidities. The Kidney Disease Improving Global Outcomes (KDIGO) guidelines recommend Basiliximab as a first-line induction therapy across all types of donor-recipient profiles to reduce acute rejection risk and allograft loss [12,13]. However, there has been a lack of randomized clinical trials comparing induction agents when recipients are receiving a maintenance immunosuppressive therapy with tacrolimus (TAC) and mycophenolic acid (MPA) with or without steroids, and the recommendations are mainly made on the basis of meta-analysis data [11,14]. Since the incidence of acute rejection is less than 10% in most reported for cause biopsies, the sample size required for randomized clinical trials that could determine small differences in observed outcomes between Basiliximab, Alemtuzumab and Thymoglobulin has been estimated to be between 1600 and 7000 [15]. Such studies would be difficult to conduct from logistics standpoints. Given such an obstacle, we reviewed 649 kidney transplant recipients at Mayo Clinic Florida from 2012 to 2016 and compared the long term kidney transplant function, based on the induction therapy used at the time of transplantation. Kidney allograft function was measured by Iothalamate as a standard clearance test [16].
After obtaining required IRB approval, all adult patients who received kidney transplant at Mayo Clinic Florida between January 2012 and December 2016 were reviewed in this study. 797 adult patients were identified; we excluded patients who received combined kidney and other solid organ transplant, kidney after previous solid organ transplant, and second or third kidney transplant. 649 patients were included in this study.
The study is a retrospective analysis of kidney transplant recipients at a single transplant center, Mayo Clinic Florida Transplant Center. Patients were divided into three groups based on the induction agent used at the time of transplantation and according to immunosuppressive therapy protocols approved and adopted by Mayo Clinic Transplant Enterprise. Group 1: Represent low immunological risk and received Basiliximab (patients 65 years of age and older and/or two haplotype matched donors or zero mismatch), Group 2: Received Alemtuzumab (patients 64 years of age and younger and/or no detected Donor Specific Antibodies (DSA) or detected DSA but with Mean Florescence Intensity (MFI) < 2000), Group 3: Represent high immunological risk and received Thymoglobulin (patients 64 years of age and younger with DSA at MFI ≥ 2000 or positive acceptable flow cytometry cross match with DSA at any value). Group 1 and 3 will receive maintenance steroid with 5 mg prednisone on daily basis. Group two will not receive maintenance steroid. All transplant recipients were maintained on TAC and MPA for long term immunosuppression.
Patients' demographic data were collected. Allografts functional data points on 4 and 48 months were collected and included: Iothalamate clearance which is a standard of care at our center and performed on all kidney transplant recipients within the first 4 months and at 48 months post-transplant. Urine protein to creatinine ratio, time to first rejection, TAC level with first rejection and pathology score of first rejection.
Categorical variables were presented as the number of subjects and the percentages. Medians were used to summarize the continuous variables. Overall comparisons among the groups for categorical variables were completed using the χ2 test. The Kruskal-Wallis test was used to compare continuous variables. The cumulative probability of a rejection was estimated using the Kaplan-Meier method. These curves were compared between groups using the log-rank test. All analyses were completed using SAS version 9.4 (Cary, NC).
Out of the 649 reviewed patients, group 1 has 149 patients received Basiliximab, group 2 has 264 received Alemtuzumab and group 3 has 236 received Thymoglobulin. Patients induced with Basiliximab were significantly older as expected. African Americans patients were represented in each group with no significant differences when compared to White patients (Table 1).
Table 1: Patients demographics. View Table 1
The protocoled 4 months Iothalamate clearance showed no difference among the three groups (Alemtuzumab 65.7 ml/min, Basiliximab 62.8 ml/min and Thymoglobulin 68.6 ml/min, p = 0.13). However, it was significantly lower in patients induced with Basiliximab at 48 months in comparison to Alemtuzumab and Thymoglobulin (p < 0.007) (Figure 1A). When we compared the three groups at 48 months after excluding all patients who received deceased kidney allografts from donors with kidney donor profile index (KDPI) greater than 85%, we found similar significant decline in allograft function at 48 months in patients induced with Basiliximab (52 ml/min) when compared with Alemtuzumab (64.0 ml/min) and Thymoglobulin (61 ml/min) (Figure 1B). Overall, 48 months proteinuria, measured by spot urine protein to creatinine ratio, among the three groups was not high. However, it was significantly higher at 48 months in patients induced with Basiliximab (0.2) when compared to patients induced with Alemtuzumab (0.1) and Thymoglobulin (0.1) (p < 0.03) (Figure 2).
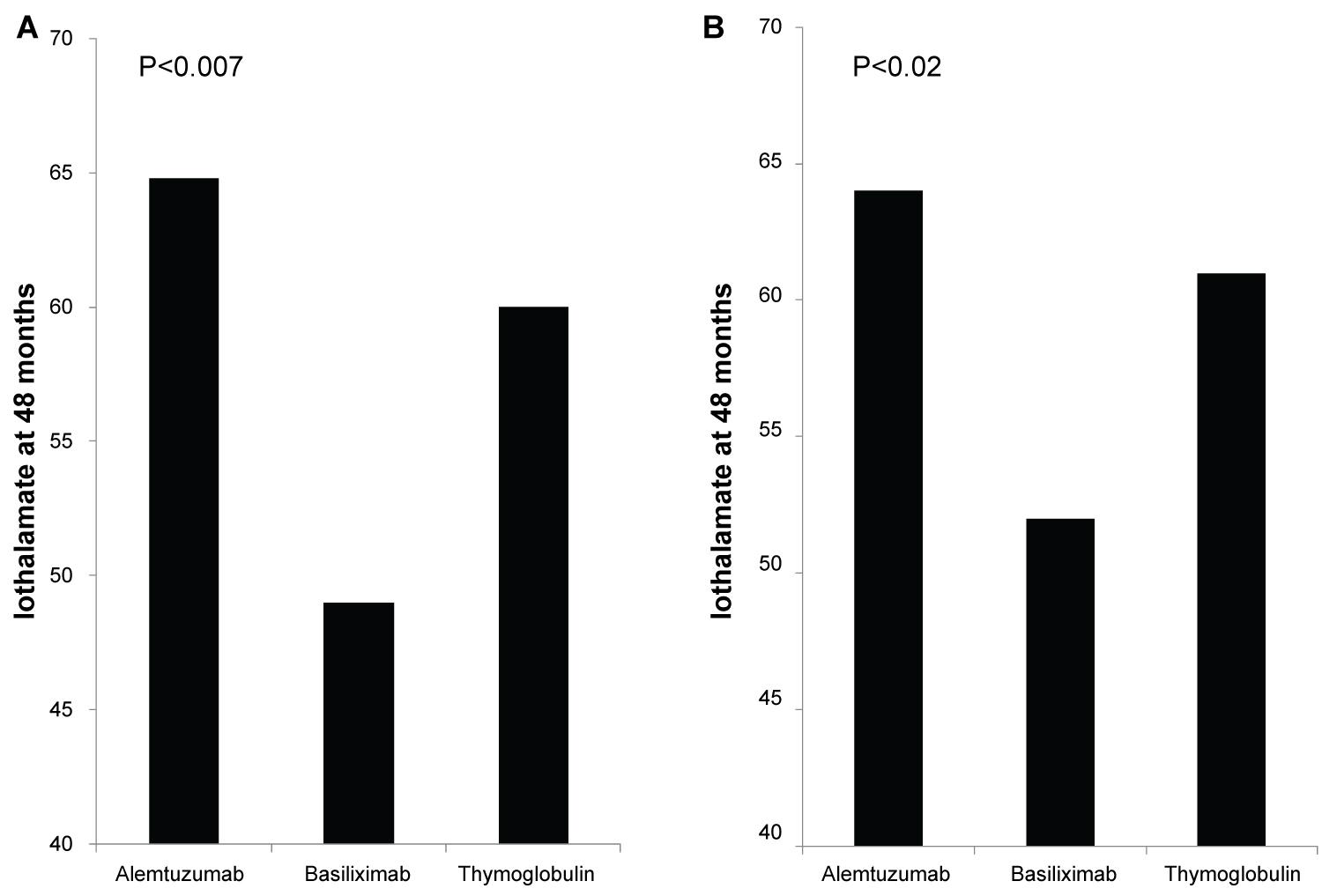 Figure 1: (A) Iothalamate clearance at 48 months, Recipients induced with Basiliximab (149 patients) showed significant decline in Iothalamate clearance at 49 when compared to Alemtuzumab (264 patients) at 64.8 and Thymoglobulin (236 patients) at 60 with p = 0.007; (B) Iothalamate clearance at 48 months, after excluding of recipients from donors with KDPI > 85%. Recipients induced with Basiliximab (129 patients) showed significant decline in Iothalamate clearance at 52 when compared to Alemtuzumab (256 patients) at 64 and Thymoglobulin (229 patients) at 61 with p = 0.02.
View Figure 1
Figure 1: (A) Iothalamate clearance at 48 months, Recipients induced with Basiliximab (149 patients) showed significant decline in Iothalamate clearance at 49 when compared to Alemtuzumab (264 patients) at 64.8 and Thymoglobulin (236 patients) at 60 with p = 0.007; (B) Iothalamate clearance at 48 months, after excluding of recipients from donors with KDPI > 85%. Recipients induced with Basiliximab (129 patients) showed significant decline in Iothalamate clearance at 52 when compared to Alemtuzumab (256 patients) at 64 and Thymoglobulin (229 patients) at 61 with p = 0.02.
View Figure 1
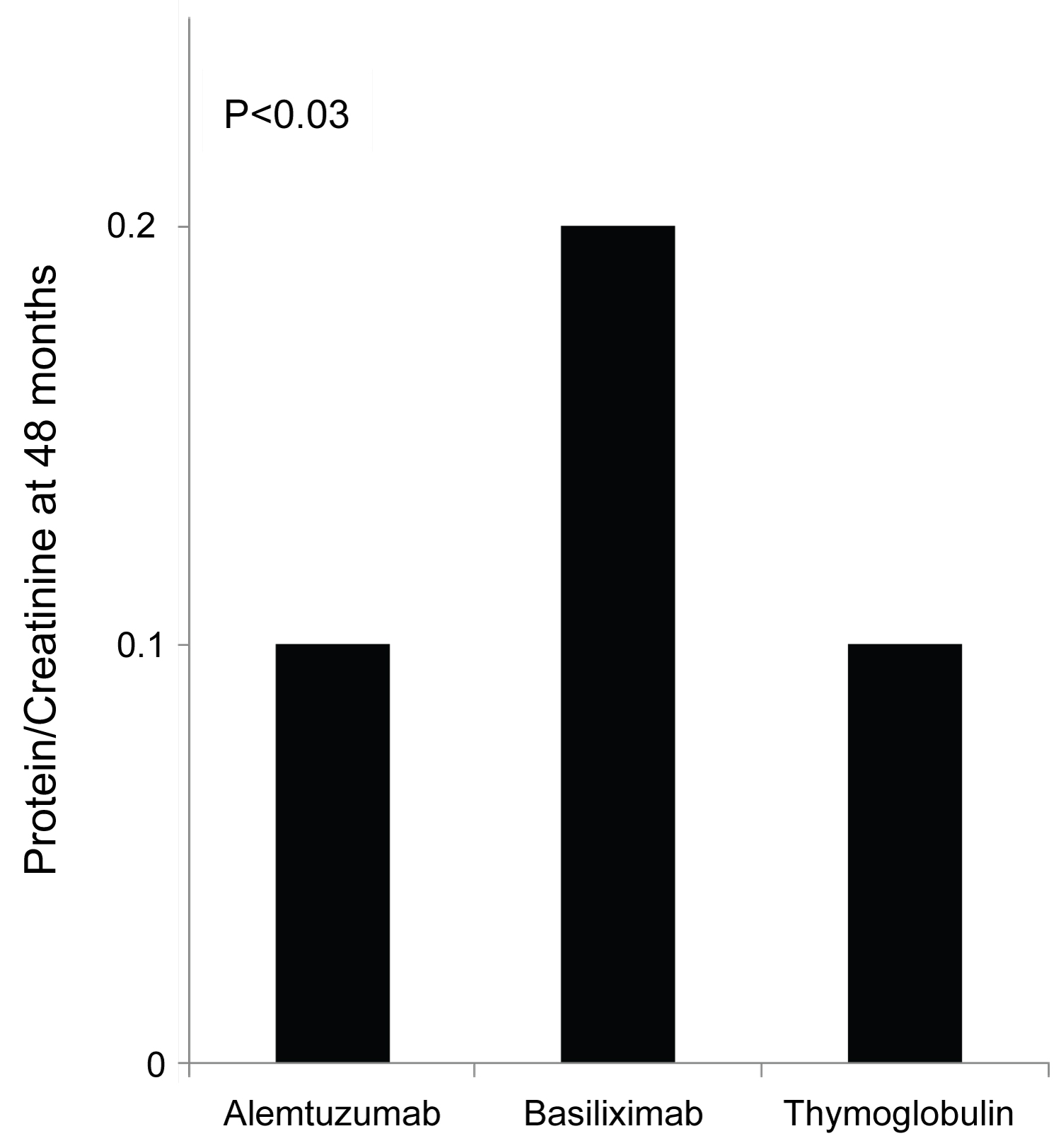 Figure 2: 48 months protein creatinine ratio measurement showed significantly elevated mean protein/creatinine when measured at 48 months at 0.2 when compared to protein/creatinine of 0.1 with both Alemtuzumab and Thymoglobulin when used for induction.
Figure 2: 48 months protein creatinine ratio measurement showed significantly elevated mean protein/creatinine when measured at 48 months at 0.2 when compared to protein/creatinine of 0.1 with both Alemtuzumab and Thymoglobulin when used for induction.
P < 0.03.
View Figure 2
Although, Banff classification of first diagnosed rejection based on protocoled and indicated biopsies was not different among the three groups, patients induced with Basiliximab showed 7.4% increase incidence of IIB rejection when compared to Alemtuzumab 2.9% and Thymoglobulin 0.0% with p < 0.3 (Table 2). Time for first rejection (Figure 3A) was not different among the three induction groups (p > 0.05) and Tacrolimus level at the time of first rejection was at 7.5 ng/mL with Thymoglobulin, 6.8 ng/mL with Alemtuzumab and 7.1 ng/mL with Basiliximab group (Figure 3B) (p > 0.05). Among the three groups of patients, incidence of viremia was higher with Thymoglobulin and viremia incidence was higher with Alemtuzumab. The overall incidence of CMV and BK was not significant among the three groups (Figure 4).
Table 2: Rejection severity. View Table 2
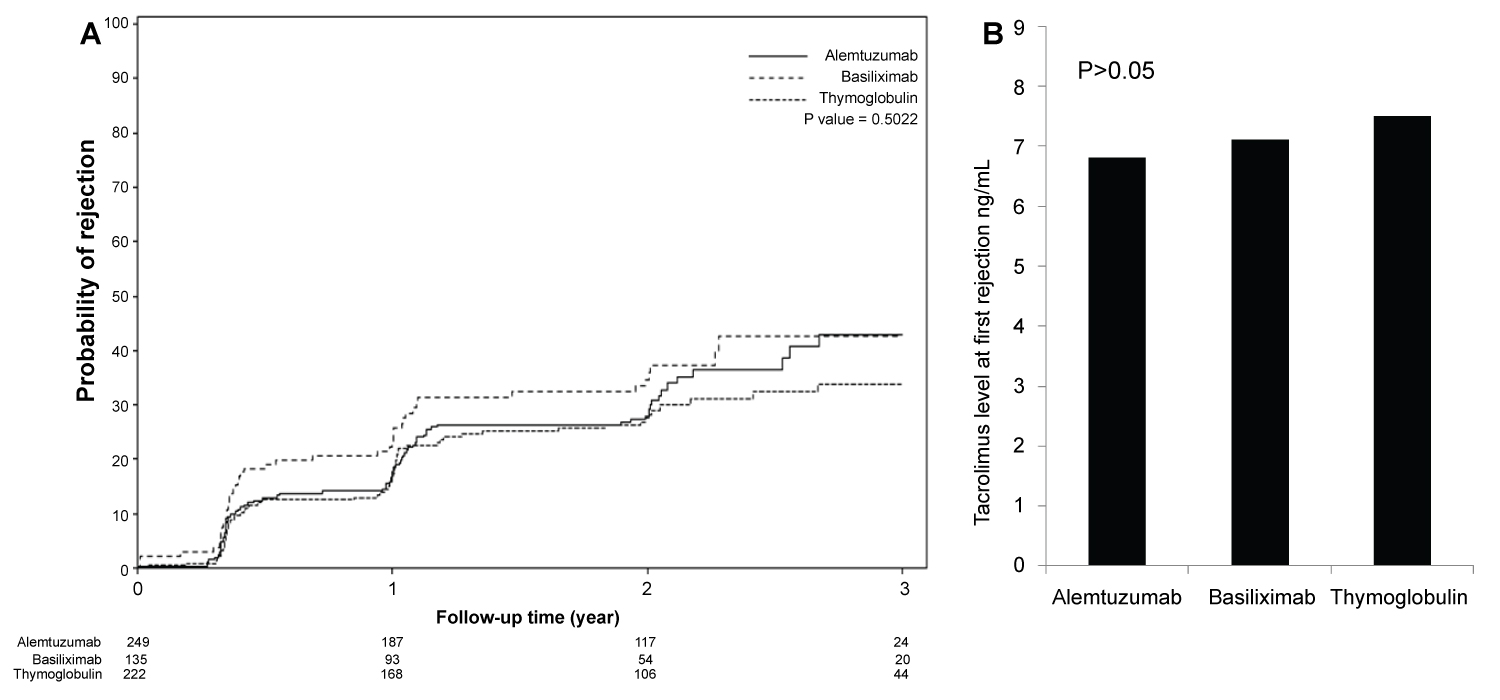 Figure 3: (A) Time to first rejection, based on protocoled or indicated biopsies, showed no significant differences among the three groups (p > 0.05); (B) Tacrolimus level checked within the 24 to 48 hours prior to first diagnosed rejection showed acceptable therapeutic levels and no differences among the three groups (p > 0.05).
View Figure 3
Figure 3: (A) Time to first rejection, based on protocoled or indicated biopsies, showed no significant differences among the three groups (p > 0.05); (B) Tacrolimus level checked within the 24 to 48 hours prior to first diagnosed rejection showed acceptable therapeutic levels and no differences among the three groups (p > 0.05).
View Figure 3
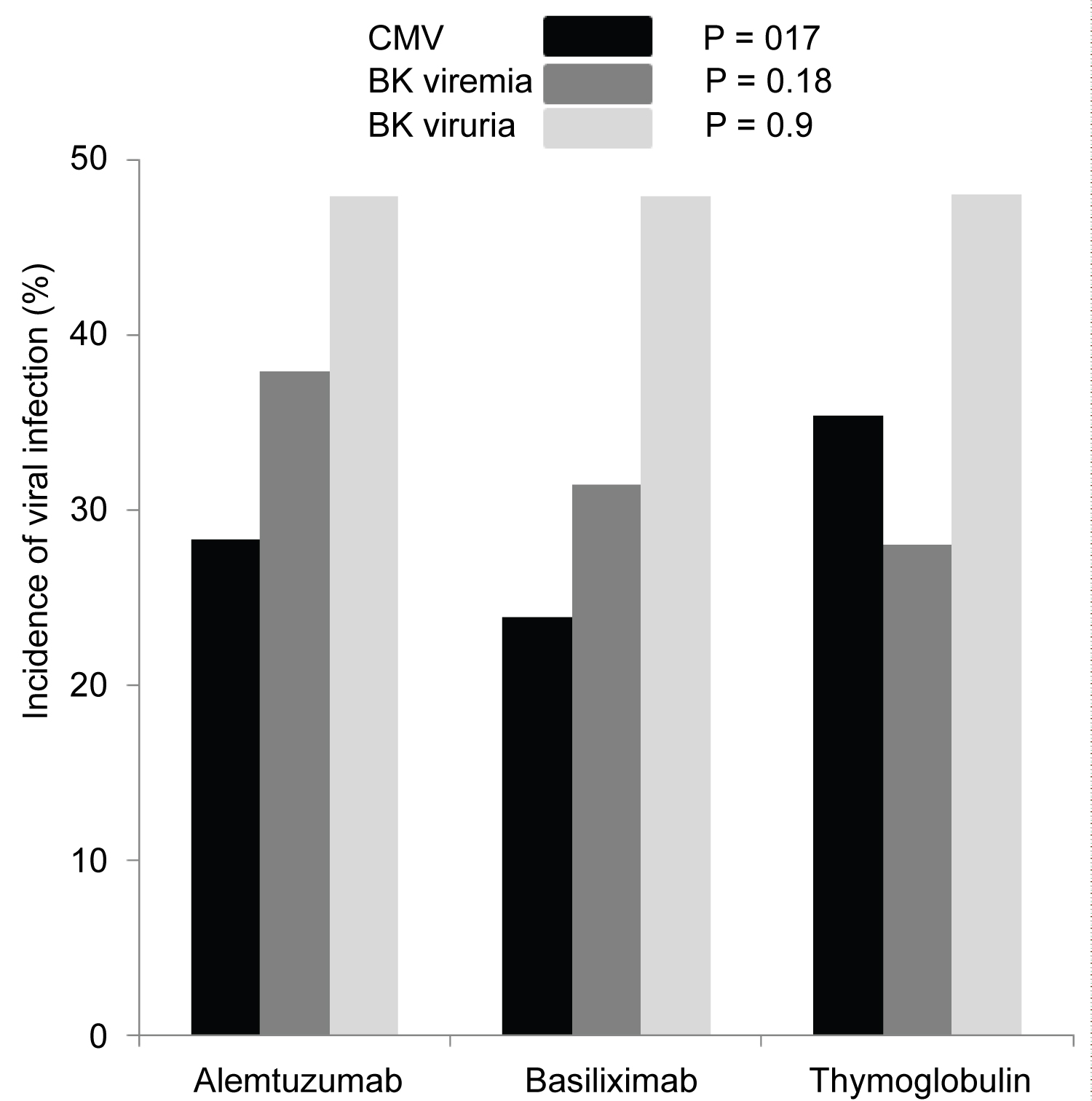 Figure 4: First diagnosed CMV viremia (▀), based on screening test and/or symptoms, was reported as percentage of patients who tested positive and showed no difference among the three groups (p=0.17). Incidence of BK viremia ( ) was higher in patients who received Alemtuzumab for induction but not significant (p=0.18). Incidence of BK viruria ( ) was similar in all patients group (p = 0.9).
View Figure 4
Figure 4: First diagnosed CMV viremia (▀), based on screening test and/or symptoms, was reported as percentage of patients who tested positive and showed no difference among the three groups (p=0.17). Incidence of BK viremia ( ) was higher in patients who received Alemtuzumab for induction but not significant (p=0.18). Incidence of BK viruria ( ) was similar in all patients group (p = 0.9).
View Figure 4
Conventionally, the monoclonal antibodies directed against the activated interleukin-2 receptor such as Basiliximab, have been usually reserved for low to moderate immunologic risk patients [17-19], whereas the depleting agents, such as Thymoglobulin and Alemtuzumab, have been used in high-risk recipients receiving kidney transplantation [7,20,21]. Our study is a retrospective analysis of kidney allograft's long term function based on immunosuppressive induction agents used at time of transplant. We found a significant drop in Iothalamate clearance when measured at 48 months post-transplant. However, the 4 months Iothalamate clearance was similar among all patients. This change from equivalent Iothalamate clearance at 4 months between the groups to a drop in Iothalamate clearance at 48 months in basiliximab group may be less related to donor factors and more to immunosuppressive approach. In order to correct for the donor factors, we repeated the 48 months analysis after excluding all recipients from all three groups who received kidney allografts from deceased donors with KDPI > 85%. The secondary analysis still showed significant drop in Iothalamate clearance in patients received Basiliximab for induction when compared to Alemtuzumab and Thymoglobulin.
The Cochrane Collaboration published a meta-analysis of randomized controlled trials that compared IL-2 receptor antagonist, such as Basiliximab, induction with placebo and Thymoglobulin [22]. Acute rejection rates were lower with IL-2 receptor antagonist versus placebo and graft loss was reduced. However, Thymoglobulin was not more effective in preventing rejection, and the safety profile favored IL-2 receptor antagonist induction. Based largely on these findings, Kidney Disease Improving Global Outcomes (KDIGO) guidelines recommended that induction therapy with a biological agent be a routine part of the initial immunosuppressive regimen and that an IL-2 receptor antagonist agent to be the first-line therapy [23]. However, little information is available about the long term effects of the IL-2 receptor antagonist Basiliximab, most of the published studies addressed the short term benefits and risks of Basiliximab in terms of reducing incidence of rejection and monitoring for side effects [17]. In our study, we reviewed the long term, five years, kidney allografts outcome when using Basiliximab vs. Alemtuzumab and Thymoglobulin for induction. We showed no difference in time to first diagnosed rejection, no difference in the severity of first diagnosed rejection based on Banff classification [24] among patients who were induced with Basiliximab when compared to Alemtuzumab and Thymoglobulin. We accounted for possible donor factors represented by high KDPI by repeating the analysis after excluding all patients who received kidney allografts from donors with KDPI > 85%. The Iothalamate clearance [25] at 5 years post-transplant was significantly lower in patients who received Basiliximab for induction when compared to Alemtuzumab and Thymoglobulin. This results remains the same even after we excluded old recipient of kidney allograft from donors with KDPI > 85%. Despite the similarities in serology and pathology findings among the three groups, the significant difference in Iothalamate clearance at 5 years post-transplant in the Basiliximab group is alarming. We could argue that the patients who received Basiliximab had chronic smoldering and subclinical cellular injury that resulted in such decline in kidney allograft function and the increase in proteinuria [26,27].
In this study, we analyzed the incidence of viral infection, mainly CMV and BK, among the 3 groups of patients based on the induction agent. Multiple studies have shown an increased risk of viral infection when T-cell depleting agent is used for induction therapy versus Basiliximab [28-30]. However, we found no significant difference in the incidence of either CMV or BKV disease among the different induction groups.
This study demonstrated potential effect of the induction agent used at the time of kidney transplantation on 5 years allograft function when measured by Iothalamate clearance. Using Basiliximab resulted in lower Iothalamate clearance and increased proteinuria 5 years post-transplant.