The surgical treatment of end-stage chronic venous insufficiency involves valvular repair or transplantation to restore venous valve function and structure. Current valve substitutes can have issues with durability, thrombogenicity, susceptibility to infection, and a lack of growth potential. Therefore, the development of tissue-engineered venous valves represents a promising solution. Before clinical use of these grafts, in vivo animal testing is necessary. Here we describe the development of different surgical techniques for tissue-engineered venous valve transplantation in a low-pressure system (venous circulation) in a large animal model. Tissue-engineered vein valves prostheses were generated using decellularized jugular ovine Vein Valves (oVV) reseeded with ovine peripheral Blood-derived Endothelial Cells (oBEC). oVV were transplanted orthotopically and heterotopically in an ovine model. Four oVV were transplanted into the jugular position (two decellularized and two decellularized/reseeded vein valves). Two decellularized oVV were heterotopically transplanted into the superior vena cava after induction of Tricuspid Valve Insufficiency (TVI). All animals survived the surgery. After three months, grafts were explanted for histological evaluation. All grafts were permeable and the vascular wall was thickened compared with the native vein. Macroscopic examination revealed loss of valve cusps in all cases. Despite a postoperative regimen of anticoagulants, intraluminal thrombi of various dimensions were found in two implants. In all cases, the histology showed a distinct cell infiltration of the vein wall. Intrathoracic implantation and increase of venous pressure in systole did not prevent venous valve alteration. These findings suggest that proposed approaches are not a suitable for in vivo testing of such grafts.
Venous valve, Decellularization, Animal model, Blood-derived endothelial cells, Venous substitution, Tricuspid regurgitation
Treatment options for patients suffering from Chronic Venous Insufficiency (CVI) are quite limited, usually comprising conservative approaches [1,2]. In end-stage CVI of the legs, the surgical treatment is the method of choice [3,4]. This involves valvular repair [5] or transplantation [6] to restore venous valve function and structure. Current valve substitutes can have issues with durability, thrombogenicity, susceptibility to infection, and a lack of growth potential. The development of tissue-engineered venous valves substitutes may be a promising solution [7]. Some tissue-engineered prostheses (pulmonary heart valves) have been clinically approved after successful animal testing [8,9]. For clinical implementation of tissue-engineered venous valves, a reliable large animal model is necessary for preclinical testing. The data on in vivo performance of tissue-engineered valvular venous grafts prostheses are still limited.
Teebken [10] described fabrication of tissue-engineered venous valves using decellularized ovine jugular veins seeded with previously-isolated recipient Endothelial Cells (EC) and myofibroblasts. One week after implantation of these grafts in the jugular position, minor signs of valvular sinus thrombosis could be detected. After 12 weeks, some grafts showed loss of valvular function and varying degrees of thrombosis.
In our previous study, a novel decellularization method of ovine jugular venous valves using a combination of two detergents at low concentration has been described [11]. The graft appeared cell-free, the extracellular matrix was preserved and the DNA-amount was significantly reduced.
The goal of presented study was in vivo testing of previously generated venous valves, using the sheep as experimental animal. For orthotopic transplantation, decellularized and decellularized/endothelialized grafts were used. To improve the oVV performance, we developed a novel surgical technique of heterotopic venous valve transplantation in a large animal model.
All experiments were approved from Lower Saxony State Office for Consumer Protection and Food Safety (LAVES) under the number 11/0635. All animals received humane care and experiments were performed in compliance with the Guide for the Care and Use of Laboratory Animals.
Ovine jugular veins were harvested under clean conditions. After adherent tissue removal, each venous segment was assessed for the number of valves and valve cusps. A cuff of the venous wall (2.0-2.5 cm on both sides of vein valve) was preserved. Vessel segments were kept for short-term storage in Phosphate-Buffered Saline (PBS) at 4 ℃. Decellularization was performed in a solution, containing a combination of 0.05% sodium deoxycholate (Sigma Aldrich) and 0.05% sodium dodecyl sulfate (Sigma Aldrich) for 24 hours at room temperature (25 ℃) under shaking conditions. To remove the residual detergent sand cell debris, the matrix was washed with sterile distilled water for 24 hours, followed by washing steps in PBS supplemented with 100 IU/ml penicillin-streptomycin and partricin for 72 h in six washing steps.
Isolation and differentiation of late outgrowth Blood-derived Endothelial Cells (oBEC) were conducted as previously described [11]. In brief, 100 ml of peripheral blood from potentially tissue-engineered venous valve recipient sheep were collected from the jugular veins. Separation of the monocyte fraction was performed by centrifugation. The resultant monocyte phase was suspended in Endothelial Cell Medium EGM-2 (Lonza, Basel, Switzerland).
Reseeding of decellularized oVV was performed in a specially-designed heart valve bioreactor system [12] that was modified for vein valve recellularization. Reendothelialization of decellularized oVV was performed as previously described [11] by seeding of 4 × 106 oBEC under static-rotational conditions for 12 h followed by proliferation and cultivation oBEC cells under pulsatile continuous flow (10 ml/min) for 5 days before being implanted in sheep.
For this study, we performed reendothelialization, using established methods, in three decellularized venous valves. Two reseeded valves were used for implantation, another one served as control prior surgery.
A total of six juvenile female black-headed sheep of about 3-months-old and weighing between 20-25 kg (22.8 kg ± 1.99) were operated on. The animals were divided into three groups according to the surgical technique and type of graft (Table 1).
Table 1: Description of experimental groups. View Table 1
All implantations were performed under general anesthesia after intubation and administration of 5000 units of heparin before the vascular procedure.
In case of orthotopic jugular vein replacement, the right jugular vein was prepared. Two vascular clamps were applied and a venous segment (3-5 cm) was resected. Proximally and distally, each end-to-end anastomosis was performed with a continuous suture using Prolene® 5-0 (Ethicon, Blue Ash, USA). Resected venous segments were substituted with the respective decellularized/reseeded (group I) or decellularized (group II) venous valve grafts (Figure 1).
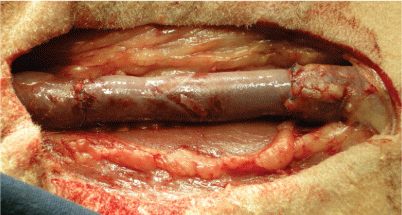 Figure 1: Implantation of vein valve in the neck position-jugular vein substitution (groups I and II). View Figure 1
Figure 1: Implantation of vein valve in the neck position-jugular vein substitution (groups I and II). View Figure 1
For intrathoracic implantation, a left antero-lateral thoracotomy was performed. The superior vena cava was prepared and a venous segment (3 cm) was resected 1.5 cm above the junction with the Right Atrium (RA). This segment was substituted with a decellularized oVV graft by using a continuous suture and end-to-end anastomoses on both sides with Prolene® 5-0 (Ethicon, Blue Ash, USA) (Figure 2). To access the tricuspidal valve, a purse-string suture on the lateral side of RA appendage was applied. Through the middle of this stitch, a small incision of RA was performed and after introducing the forceps, one tricuspid valve leaflet was gripped and, using scissors, resected. This defect was performed under continuous observation with Transesophageal Echocardiography (TEE) in order to achieve a mild Tricuspid Valve Insufficiency (TVI) (Figure 3).
 Figure 2: Implantation of decellularized vein valve in the thorax position-substitution of superior vena cava with induced tricuspid valve insufficiency (group III). View Figure 2
Figure 2: Implantation of decellularized vein valve in the thorax position-substitution of superior vena cava with induced tricuspid valve insufficiency (group III). View Figure 2
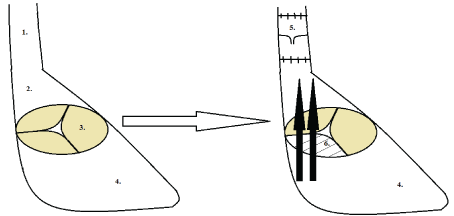 Figure 3: 1) Schema of substitution of superior vena cava 2) Right atrium, 3) Intact tricuspid valve, 4) Right ventricle; 5) A decellularized vein valve and 6) Induced tricuspid valve insufficiency. View Figure 3
Figure 3: 1) Schema of substitution of superior vena cava 2) Right atrium, 3) Intact tricuspid valve, 4) Right ventricle; 5) A decellularized vein valve and 6) Induced tricuspid valve insufficiency. View Figure 3
All animals received antithrombotic treatment with daily subcutaneous administration of Enoxaparin sodium (1 mg/kg) for two weeks following surgery.
Grafts explantation was performed 3 months after implantation surgery. Upon explantation, each graft was gently prepared and cleaned from surrounding tissues.
Venous valves were fixed in formalin and embedded in paraffin. For histological staining, 7 μm slices were cut, deparaffinized, and either Hematoxylin and Eosin (H&E) or Pappenheim stained.
Frozen explants were cutin 5 μm sections. Tissues were blocked with donkey serum (1:10, Sigma-Aldrich Chemie GmbH, St. Louis, USA), incubated with primary antibody (anti-CD45 IgG1, 1:100, AbDSerotec, Raleigh, USA), extensively washed with PBS, and incubated with the secondary antibody (anti-donkey IgG Cy3, 1:300, Dianova GmbH, New York, USA). After washing with PBS, the nuclei were stained with DAPI (Life Technologies, Carlsbad, USA) and mounted in Fluorescent Mounting Medium (Dako, Glostrup, Denmark). Ovine lymph nodes were used as a positive control.
Sheep body weight was described as the mean ± the standard deviation. Cell counting in three successive histological slices (HE staining) of each explants graft were performed using Image J 1.48 v (National Institutes of Health, USA). Cell number from groups I and II (different grafts, same implantation location), as well as those from groups II and III (same grafts, different implantation location) were compared. Results were analyzed using t-test in XLSTAT (Version 19.1 for Windows, Addinsoft, NY, USA) and expressed as the mean ± the standard deviation. The results were considered statistical significant at p < 0.05.
After decellularization, a cell-free extracellular venous valve matrix with intact valve cusps was obtained (Figure 4). Reseeding of decellularized vein valves with oBEC in a bioreactor system showed nearly complete cell coverage of leaflets, sinus and the vascular wall.
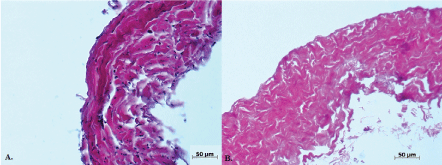 Figure 4: Native (A) and decellularized (B) vein wall. View Figure 4
Figure 4: Native (A) and decellularized (B) vein wall. View Figure 4
During oVV transplantation, as well as postoperatively, no major complications were observed. All animals survived until explantation.
For histological and immunohistochemical analyses, the venous valve graft was cut in two longitudinal pieces between valvular commissures. All explanted grafts were permeable. However, the lumen of one explanted graft from group I was partially obstructed due to neointima formation. This finding did not compromise the blood return through the jugular vein. The animal showed no signs of upper venous obstruction.
All explants showed loss of valvular leaflets and thickening of the vascular wall (Figure 5). The remnant leaflet morphology is described in Table 2.
Table 2: Morphological description of explanted venous valve leaflet remnants. View Table 2
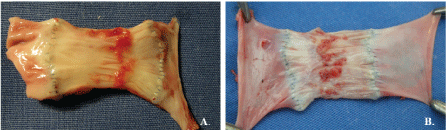 Figure 5: Retraction of vein valve cusps after 3 months (jugular vein position). A) Decellularized vein valve and B) Decellularized/reseeded vein valve. In both cases, the cusps were thickened, short, and hadred-colored solid deposits. View Figure 5
Figure 5: Retraction of vein valve cusps after 3 months (jugular vein position). A) Decellularized vein valve and B) Decellularized/reseeded vein valve. In both cases, the cusps were thickened, short, and hadred-colored solid deposits. View Figure 5
Despite daily administration of anticoagulants after surgery, thrombi of various sizes were detected (Figure 6). Thrombus localization was predominantly in the region of valvular sinus. In one explanted graft from group II, a thrombus on the entire implant length was detected, but without complete lumen obstruction.
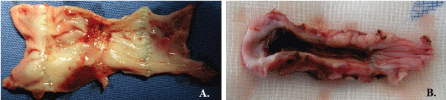 Figure 6: H&E staining if intraluminal thrombi. A) Thrombosis of valvular sinus-animal from group III and B) Thrombus on the entire implant length (animal from group II). View Figure 6
Figure 6: H&E staining if intraluminal thrombi. A) Thrombosis of valvular sinus-animal from group III and B) Thrombus on the entire implant length (animal from group II). View Figure 6
The vascular anastomosis showed no signs of stenosis. After 3 months, the sutures were completely covered. No thrombi were detected.
Right ventricles of explanted hearts from animals in group III were not dilated. The free wall revealed normal thickness.
Using H&E staining, a high concentration of nuclei in the vascular wall, as well as neointima formation could be confirmed (Figure 7). The venous valve appeared degenerated in all explants. In the valvular sinus of some grafts, thrombi of different sizes were detected (Figure 8). Using Pappenheim staining, various degrees of leukocyte infiltration of venous wall were observed (Figure 9).
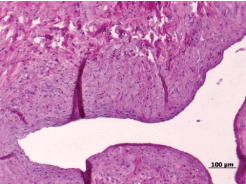 Figure 7: Venous wall thickening and neointima formation (animals from group I). View Figure 7
Figure 7: Venous wall thickening and neointima formation (animals from group I). View Figure 7
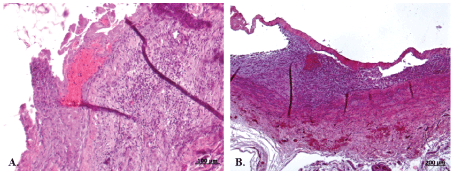 Figure 8: Degenerated venous valve with a fresh A) and Organized B) thrombi in valvular sinus. View Figure 8
Figure 8: Degenerated venous valve with a fresh A) and Organized B) thrombi in valvular sinus. View Figure 8
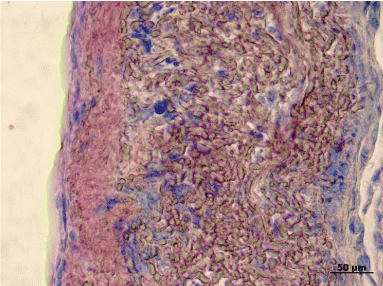 Figure 9: Pappenheim-staining showing leukocyte infiltration of the venous wall. View Figure 9
Figure 9: Pappenheim-staining showing leukocyte infiltration of the venous wall. View Figure 9
Vein wall cell infiltration was significantly higher in group II, compared to group I (p = 0.012) and group III (p = 0.006) (Figure 10).
 Figure 10: Cell number in the explanted grafts was significantly higher in group II, compared to group I (p = 0.012) and group III (p = 0.006). View Figure 10
Figure 10: Cell number in the explanted grafts was significantly higher in group II, compared to group I (p = 0.012) and group III (p = 0.006). View Figure 10
In all explants, CD45-positive cells were detected (Figure 11).
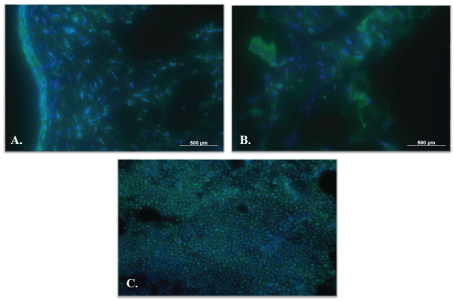 Figure 11: CD45-positive cells (green). A) Decellularized graft; B) Decellularized/reseeded graft and C) Ovine lymph node. Cell nuclei are stained with DAPI (blue). View Figure 11
Figure 11: CD45-positive cells (green). A) Decellularized graft; B) Decellularized/reseeded graft and C) Ovine lymph node. Cell nuclei are stained with DAPI (blue). View Figure 11
In this study, we performed in vivo testing of tissue-engineered venous valvular grafts in a sheep model, using different approaches for transplantation.
The efficacy of detergent combination, sodium deoxycholate and sodium dodecyl sulfate, used for heart valve decellularization, was demonstrated at clinical and experimental level [8,9]. To diminish the potential negative toxic effect on the extracellular venous matrix, produced by these chemicals, a low-concentrated detergent solution was used in this study. The novelty of our study consists in the treatment of native veins with low-concentrated detergent solution (ten times less than used for heart valves decellularization). The decellularization of jugular oVV resulted in a significant loss of cells and nuclei, as well as reducing of DNA [11]. These valves were reseeded using progenitor oBEC. Previous cellular reseeding of decellularized venous valve matrices showed an improvement of valve performance and durability at least 6 month after femoral vein replacement in sheep [13]. In the aforementioned ovine model, these prostheses showed no major signs of thrombosis even in the absence of antithrombotic medication. In our model, cell-free matrices have been reseeded with autologous outgrowth endothelial cells that were isolated and differentiated from the peripheral blood of the animal. Two different in vitro venous valve reendothelialization approaches, described previously by our group, demonstrated a quite completely endothelial cell covering of venous leaflets and venous wall and no significant differences between both reseeding paths [11]. Despite complete reendothelialization, the grafts from group I demonstrated minor thrombosis signs. Also, histology of explanted grafts revealed a partial loss of endothelium. We assume, that preseeded endothelial layer is not stable in vivo and detached after implantation. Low flow environment, as well as particularities of ovine coagulation system (thrombocytosis) could lead to thrombus formation.
Despite reendothelialization and administration of anticoagulants, some animals developed a degree of thrombosis after 3 months when oVV were implanted in the jugular position. One graft, implanted in an intrathoracal position, showed only minor signs of thrombosis in valvular sinus region.
Various animal models for preclinical testing of tissue-engineered venous valves have been proposed. Initial attempts to replace the jugular vein with a glutaraldehyde-fixed human venous valve in a canine model demonstrated thrombotic graft occlusion after one week [14]. Another model, involving implantation of a mechanical venous valve prosthesis in a canine femoral vein, showed sufficient valve performance up to 3 months and prosthesis occlusion due to neointima formation after 2 years [15]. Decellularized venous valves, implanted in the jugular vein, showed loss of valvular function and different signs of thrombosis 6 weeks postoperatively [10].
Because of their physiological similarities to humans, implantation suitability, as well as the possibility of long-term observation, sheep are considered an appropriate experimental animal for preclinical testing of cardiovascular implants [16]. Sheep models also provide an optimal environment for endothelialization [16]. In our experiments, two different endothelialization methods were investigated-in vitro EC reseeding before implantation and in vivo implantation without endothelialization, known as guided-tissue regeneration.
Because of their similarities with femoral and great saphenous veins [17], jugular vein substitution has been accepted as model to test venous valves prosthesis [10,18]. Ovine jugular veins usually possess 1 to 3 valves, which are normally bicuspid. Valve function involves closing and avoiding blood return during head deflection. Jugular venous valve substitution has been performed in our experiments using decellularized and preseeded oVV conduits.
Teebken, et al. [10] successfully performed autologous venous valve transplantation in 8 sheep. After 12 weeks, sufficient valve function and patency of all grafts was confirmed. Taking into account these animal experiments, we intentionally avoided having native auto grafts as a control group.
After 3 months, all grafts of group I and II showed disappearance of the valve cusps. Graft obstruction (group II) and different degrees of thrombosis were comparable with the previously described results [10]. Our histological analyses confirmed the presence of valvular destruction and thrombosis, as well as high cellular infiltrations. We assumed that low pressure in the venous system and lack of pulsatility was the major factors altering the "open-close" venous valvular function. Low blood flow via the graft leads to thrombosis and migration of blood leukocytes into the venous wall, confirmed by the presence of CD45-positive cells. External compression of the neck (e.g. via bandage of the postoperative scar or lying of the head during sleep) could be a factor that contributes to a valvular function failure.
Because of these findings, a novel surgical model has been developed and applied. Vena cava substitution was chosen to avoid head deflection or other external obstruction factors. To increase the graft pulsatility, an increase of reversal venous blood pressure was achieved in cardiac systole by creating a mild tricuspid valve insufficiency. Using this manoeuver, an important blood quantity returns during right ventricle systole, thus improving the "open-close" function of the venous valve. However, this manipulation on tricuspid valve did not lead to improvement of oVV durability. Percutaneously-induced tricuspid valve insufficiency has been described in sheep, using papillary muscle avulsion [19]. These experiments were performed in a small number of animals as model for transcatheter valve implantation in tricuspid or mitral regurgitation. All sheep died directly after induced insufficiency because of major hemodynamic complications. In contrast, in our model, moderate tricuspid regurgitation was induced by creation of a defect in one leaflet under TEE monitoring which assured animals’ survival.
Unfortunately, maintenance of venous valve structure and function in this animal model was not improved after induced tricuspid insufficiency. After 3 months, the results were similar to jugular vein substitution. Additionally, cellular infiltration, as well as CD45-positive cells in the vascular wall could be found in our explants.
CD45 (leukocyte common antigen) is expressed in almost hematopoietic cells, except mature erythrocytes [20]. Our finding of CD45-positive cells in the wall of explanted venous grafts, including the results of Pappenheim staining, indicates that the inflammation is related to graft remodeling.
Vascular wall remodeling via inflammation is considered to be beneficial in maturation of tissue-engineered vessels after implantation in the venous system [21]. Roh, et al. [21] demonstrated that bone marrow mononuclear cells, seeded on tissue-engineered grafts, cause migration of monocytes from the surrounding tissues and their self-differentiation. This phenomena was not observed after carotid artery substitution with decellularized veins [22]. We assume that venous wall leukocyte infiltration, which was observed in our study, could be a part of the inflammation-mediated vascular remodeling and is dependent on blood flow.
Nevertheless, re-endothelialized grafts showed lower venous wall cell infiltration, thus confirming that major inflammation cells migrate from surrounding tissues.
The presence of thrombus, despite postoperative anticoagulants administration, underscores the need for a better understanding of the sheep blood coagulation system. In a study based on 47 observations on adult sheep, an increased number of leukocytes and thrombocytes in sheep blood compared to human blood could be confirmed [23]. This factor could explain the presence of leukocytes in the venous wall as well as thrombus formation.
The animal number in each group was small and this could be a study limitation. However, according to the requirement, expressed in authorization of animal protection, we interrupted our in vivo experiences as soon as proposed models were non-functional.
The proposed models to test venous valve in sheep are not reasonable. The decelerate blood flow through the jugular vein lumen, loss of reseeded endothelial cells and ovine blood particularities (elevated number of thrombocytes) conduct primary to graft thrombosis and secondary to venous valve dissolution. The model, described by Yuan, et al. [13], consisting in femoral vein replacement in sheep, could serve as an appropriate model for in vivo testing of tissue engineered venous valves. In this situation, the augmented blood flow through the graft, combined with preserved low-leg muscle pump function, provide improved conditions for the venous valve function.
From our venous jugular valve replacement experiments, we can conclude that the proposed approaches are not an appropriate for preclinical testing of tissue-engineered venous valve grafts. Although we implanted the jugular vein graft inside of thoracic cavity to exclude external factors of compression, and additionally attempted to increase the "open-close" function of the valve by increasing blood return, graft pulsatility, and venous blood pressure by inducing moderate TVI, the maintenance of the oVV function was not be achieved. It seems that orthotopic and heterotopic jugular vein replacement in sheep is not an appropriate large animal model for testing on oVV function and durability in venous low-pressure system environment.
In summary, in the present study, we demonstrated the unsuitability of jugular and superior vena cava replacement in sheep model for testing of tissue-engineered venous valve grafts. On the other hand, the model, consisting in femoral vein replacement in sheep, by preserving of low-leg muscle pump function, may provide improved conditions for venous valve durability. This approach, could serve as an appropriate model for in vivo testing of such kind of grafts.
This work was supported by the CORTISS foundation. We are grateful to Rosalinde Katt, Astrid Diers-Ketterkat, Karin Peschel, Doreen Unger, Petra Ziehme and Tobias Goecke for their technical assistance. We would like to thank Erin Boyle for editing of the manuscript.
The authors declare no conflict of interest.