The systemic toxic effects of di-isononyl phthalate (DINP) were evaluated in a 4-wk study in male Sprague-Dawley rats. The animals were administered DINP intravenously at dosages of 125, 250, and 500 mg/kg every other day for 4 wk. The control and the positive control group were administered vehicle (egg yolk phosphatides plus glycerol solutions) and 500 mg/kg di(2-ethylhexyl) phthalate (DEHP), respectively. Clinical signs were observed immediately after administration and consumption of food, and mean body weight was recorded once a week for 4 wk. The absolute and relative organ weights, hematology, clinical chemistry, and pathological changes were evaluated at the end of the study.
No adverse effects nor death were observed in any animal in the DINP-treated groups. Food consumption, mean body weight, and absolute and relative organ weights of DINP-treated rats were not significantly different from those of the control group. Hematological parameters (LYMPH%, NEU%, and NEU#) in the 500 mg/kg DINP-treated group increased significantly, whereas no treatment-related changes in clinical chemistry parameters were observed in any DINP-treated animals. Lesions were observed in the liver and testis of rats treated with 500 mg/kg. In this 4-wk study, the no observed adverse effect level (NOAEL) of DINP was 250 mg/kg, based on the pathological changes in the liver and testis and the significant changes in hematology observed in the 500 mg/kg DINP-treated group.
Treatment related abnormalities, consisting of obvious pathological changes in the liver, kidney and testis, increases in hematological parameters, and increases in clinical chemistry parameters, were observed in the 500 mg/kg DEHP-treated group, which served as the positive control.
Di-isononyl phthalate (DINP), Repeated dose toxicity, Intravenous infusions, Di(2-ethylhexyl)phthalate (DEHP)
As a general-purpose plasticizer, di-isononyl phthalate (DINP) has been widely used in the production of a series of polyvinyl chloride (PVC) plastic products, including building materials, gloves, adhesives, toys, furniture, and medical devices. DINP is often not bound to the plastics and can leach into the surrounding environment, then enter human bodies, becoming a potential public health risk. For this reason, the safety of DINP has been scrutinized in recent decades. Although previous toxicological data suggested the potential risk posed by DINP exposure, a systemic analysis from the American Council on Science and Health (ACSH), the NTP Center for the Evaluation of Risks to Human Reproduction (NTP-CERHR) [1], and other expert groups [2-4] all supported the conclusion that the health risk of DINP was low and that there was no convincing evidence of adverse effects in humans with normal use. Considering the benefits of DINP and other phthalates and the lack of comprehensive toxicological information on substitute compounds, additional toxicological information regarding DINP will be useful.
Humans are exposed to DINP in various ways, such as ingestion, inhalation, and dermal absorption. Among these, ingestion is the most common. Consequently, current toxicological research is often focused on the systemic toxicity of DINP when administered orally [5-8]. However, few studies have focused on the adverse effects of DINP after exposure by other routes, including by intravascular administration, which exposes it directly to the blood. Nevertheless, when DINP is used as a plasticizer in blood storage bags and medical tubing, it may leach out of these devices and enter the blood directly [9]. To obtain more information about the potential toxicological effects of DINP, we evaluated the subchronic effects of DINP when given by intravenous (i.v.) injection every other day for 4 wk in male Sprague-Dawley rats.
DINP (CAS 68515-48-0), DEHP (CAS 117-81-7), egg yolk phosphatides, and glycerol were purchased from ZHUHAI UNICIZERS INDUSTRIAL CO. LTD, Sigma, Germany Lipoid GmbH, and SHANTOU ZIGUANG AMINO ACIDS CO. LTD, respectively. The purity of the egg yolk phosphatides was above 80% and the purity of the other three chemicals was above 99%.
Male Sprague-Dawley rats (6-8 wk old) were purchased from Vital River Laboratory Animal Inc. (Beijing, China) and housed under specific pathogen-free (SPF) conditions. They were housed in clear polycarbonate cages with wood chips for bedding and given a pellet rodent diet and water ad libitum. The animals were maintained under controlled conditions (22 ± 2 ℃, humidity 50 ± 10%, and 12-h light/dark cycle) and were examined for any clinical signs. In addition, they were weighed at predetermined intervals. After 1 wk of acclimatization, animals were randomly allocated to five groups based on their body weight (n = 10 each): Control group (egg yolk phosphatides plus glycerol solutions); 125, 250, and 500 mg/kg DINP-treated groups; and 500 mg/kg DEHP-treated group. All experimental protocols were approved by the Institutional Animal Care and Use Committee (IACUC number: IACUC-2013-014, National Beijing Center for Drug Safety Evaluation and Research, Beijing, China).
The DINP and DEHP emulsions used in this study were prepared as previously reported [10,11]. First, DEHP and DINP (10.0% w/v) were 1.2% diluted in the fractionated egg yolk phosphatides (oil phase). Before administration, the oil phase was mixed with glycerol (1.2% w/v) using high-speed cutting (10000 r/min, 10 min). A vehicle, without DEHP and DINP, was prepared similarly and served as the control solution. Thereafter, the above-mentioned mixture was further emulsified to form the final emulsion, which was diluted with water for injection at the appropriate concentration for each dose group. The control solution was diluted with the same volume of 1.2% egg yolk phosphatides and 1.2% glycerol solution and diluted with distilled water. The mean diameter of DINP and DEHP particles in the emulsion was 179.5 nm and 184 nm, respectively, as determined by a laser diffraction sizer. The mean zeta potential was -24.5 mv and -27.4 mv, respectively, as determined by a Nano ZS90 Zetasizer (Malvern Co., UK). Three days before the study began, the rats were etherized and a venous cannula was permanently inserted into the jugular vein. After surgery, the rats were raised individually, free to eat and drink. Three days later, the animals received treatment (vehicle; 125, 250, or and 500 mg/kg DINP; or 500 mg/kg DEHP) every other day for 4 wk by a 3 h iv infusion (Model BT100L, Lead Fluid Technology, Co Ltd, China) at a rate of 1.0 mL/h.
The animals were observed for any immediate toxic signs and were examined throughout the observation period to record any delayed acute effects and mortality. The animals were observed once each day after administering the test chemicals for 4 wk. All rats were weighed and dietary consumption was measured once a week (4 wk repeated study). At the end of the study, under anesthesia, the brain, heart, lung, liver, kidneys, adrenal glands, spleen, testes, and epididymis were weighed, and organ to body weight ratios were calculated.
At the end of the last infusion, a 1-mL sample of blood was drawn from the eye socket of the animals and the blood samples were examined for hematological analysis. The red blood cell (RBC) count, hemoglobin (Hb) concentration, hematocrit (Ht), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), platelet count (Plat), mean platelet volume (MPV), platelet distribution width (PDW), and red cell distribution width (RDW) were determined using JT-IR HA using a hematology analyzer (Coulter Counter Electronics, USA). Reticulocyte count (RCT) was tested using a reticulocyte count meter R-500 (Sysmex, Japan) and white blood cell differential count (WBC-DC) was determined by scopy. Lymphocytes (LYMPH%), monocytes (MONO%), neutrophils (NEU%), eosinophils (EO%), and basophils (BA%) were examined using a Multiparameter Blood Cell Counter (JT-IR, USA). For serum biochemistry analysis, blood samples were centrifuged at 2500 × g for 10 min within 1 h after collection. The sera were stored at -80 ℃ in a freezer before analysis. The serum biochemistry parameters, including calcium (Ca), potassium (K), sodium (Na), albumin (Alb), blood urea nitrogen (BUN), cholesterol, triglycerides (TG), creatinine, glucose (Glu), total cholesterol, total bilirubin, total protein, alkaline phosphatase (ALP), glutamate pyruvate (GPT), glutamate oxaloacetate transaminase (GOT), and g-glutamyl transferase (GGT) were measured using an automatic biochemistry analyzer (model Hitachi 7180, Hitachi, Japan).
Immediately after the drawing of blood, rats were deeply anesthetized with chloral hydrate. The brain, heart, lungs, liver, spleen, kidney, adrenal gland, prostate, testis, and epididymis were removed, freed from fat, and weighed. The tissues of the removed organs were fixed with 10% formalin solution, then dehydrated by ethanol step by step and embedded in paraffin wax. Paraffin sections were stained with hematoxylin-eosin (HE) and examined under a light microscope.
Data are expressed as mean ± SD. All statistical analyses were carried out using SPSS 16.0 software. The comparisons among groups were performed using one-way ANOVA. P values less than 0.05 were considered statistically significant.
One animal in the 500 mg/kg DEHP-treated group died 10 d after treatment began without any apparent gross abnormalities. No rats exhibited abnormal behaviors. There were no ophthalmological findings in any of the treated or control groups. The average body weight of the animals in the 500 mg/kg DEHP-treated group was significantly lower 21 d and 28 d (P < 0.05/0.01) after treatment began than that of the control group (Table 1). However, there were no obvious differences in food consumption between the treated groups and the control group during the study (data not shown).
Table 1: Body weight changes after i.v. administration of different doses of DINP and other test materials in male Sprague-Dawley rats for 4 wk. View Table 1
As shown in Table 2, by the end of the study, compared with the control group, the weights of the brain, heart, lung, liver, kidneys, adrenal glands, spleen, testes, and epididymis in the groups treated with different doses of DINP and the group treated with 500 mg/kg DEHP were not significantly different. In contrast, the relative organ weights of the lung, liver, and kidneys in the group treated with 500 mg/kg of DEHP were significantly greater (P < 0.05/0.01) than those of the control group.
Table 2: Absolute and relative organ weights in male Sprague-Dawley rats after repeated iv infusions of DINP and other test materials for 4 wk. View Table 2
Compared with the control group, WBC, PDW%, MONO#, MONO#, LYMPH#, and NEU# were significantly increased in the 500 mg/kg DEHP-treated group (P < 0.05/0.01) compared to the other groups. In addition, LYMPH%, NEU%, and NEU# in the 500 mg/kg DINP-treated group significantly increased (P < 0.05/0.01) compared to those in the other groups. Furthermore, the PDW%, RBC, HGB, HCT, MCHC, and MONO% were higher, whereas the RET and RET# were lower, in the 125 mg/kg DINP-treated group compared to those of the control group (P < 0.05/0.01) (Table 3).
Table 3: Hematological values in male Sprague-Dawley rats when administered DINP and other test materials repeatedly for 4 wk. View Table 3
There was a significant increase in TP, TG, Alb, and Cl levels (P < 0.05/0.01) in the 500 mg/kg DEHP-treated group. In addition, Ca increased and Glu decreased in the 125 mg/kg DINP-treated group compared to that in the control group (P < 0.05/0.01) (Table 4).
Table 4: Clinical biochemistry in male Sprague-Dawley rats when administered DINP and other test materials repeatedly for 4 wk. View Table 4
As shown in Table 5 and Figure 1, slight regional lymphocyte infiltration was seen in the kidney tissue of some animals in all the groups; however, it was seen in more animals in the 500 mg/kg DEHP-treated group (4/9) than in the control (1/10) and 500 mg/kg DINP-treated (1/10) group. Moreover, mild small local nephritic necrosis appeared in the 500 mg/kg DEHP-treated group (4/9). Considering that slight regional lymphocyte infiltration in the kidney is a common spontaneous pathological change, it may be not related to the toxic effect of DINP treatment. However, the higher number of animals with slight regional lymphocyte infiltration and small local nephritic necrosis suggested that abnormal pathological changes may be caused by treatment in the 500 mg/kg DEHP-treated group.
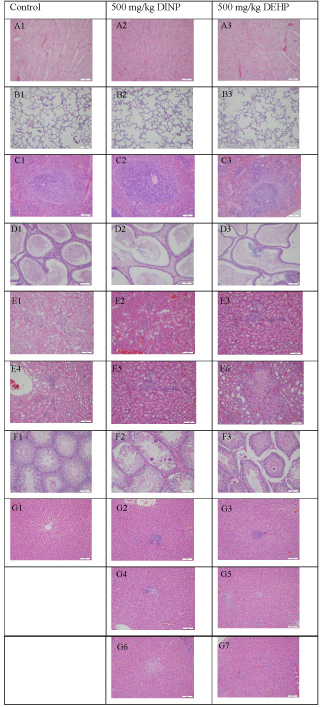 Figure 1: Histopathology of the primary organs of male Sprague-Dawley rats after repeated iv injections of DINP and other test materials for 4 wk.
Figure 1: Histopathology of the primary organs of male Sprague-Dawley rats after repeated iv injections of DINP and other test materials for 4 wk.
A) Heart; B) Lung; C) Spleen; D) Epididymis; E) Kidney; F) Testicle; G) Liver
There were no abnormal changes in the heart (A1-A3); Lung (B1-B3); Spleen (C1-C3), or Epididymis (D1-D3). Abnormal pathological changes are shown in the following primary organs: Regional lymphocyte infiltration in the kidney (E3-E5); Small local nephritic necrosis (E6); Testis local seminiferous tubule epithelium spermatocyte and spermatid disappearance (F2-F3); Liver header lymphocyte infiltration (G2-G3), tiny hepatic granulomas (G4-G5), and small local hepatic necrosis (G6-G7).
View Figure 1
Table 5: Pathological incidence in male Sprague-Dawley rats after repeated iv injections of DINP and other test materials for 4 wk. View Table 5
Obvious pathological changes were observed in the liver and testis of the 500 mg/kg DEHP- and 500 mg/kg DINP-treated groups. Microgranulomas (2/10, 500 mg/kg DINP; 4/9, 500 mg/kg DEHP), lymphocyte cellular infiltration in the header zone (3/10, 500 mg/kg DINP; 6/9, 500 mg/kg DEHP), hepatocyte stove necrosis (1/10, 500 mg/kg DINP; 1/9, 500 mg/kg DEHP), liver vacuolation (3/9, 500 mg/kg DEHP), and the disappearance of epithelium spermatocytes and spermatoblasts in the partly seminiferous tubule (1/10, 500 mg/kg DINP; 4/9, 500 mg/kg DEHP) were the primary abnormal symptoms in the testis. In addition, although the number of animals with pathological changes in the 500 mg/kg DEHP-treated group was higher than in the 500 mg/kg DINP-treated group, there were no statistically significant differences in the incidence of these changes between the two treatment groups (Table 5 and Figure 1).
DINP and DEHP are phthalates and have physicochemical similarities. As a result, the effects of general exposure to DINP may be quite similar to those of exposure to DEHP. The toxicological effects of repeated infusions of DEHP in rats has been reported in detail and the corresponding results and evaluation methods have been confirmed and quoted by the U.S. Food and Drug Administration (FDA) in the evaluation of the potential risk of DEHP [12] To investigate the toxicity of DINP when administrated intravenously, this study used similar toxicological methods to those reported in the evaluation of DEHP [11]. In addition, DEHP was chosen as a positive control.
In this study, the male Sprague-Dawley rats exposed to 500 mg/kg of DEHP exhibited obvious pathological changes in the kidney, liver, and testis. Increases were observed in hematological parameters (MONO#, NEUT%, and other inflammatory cells) and in clinical chemistry parameters (TP, TG, Alb, and CK). Those treatment-related changes were also observed in previous studies of DEHP [11,13,14] and further validated the methodology of this study. Because the body weight of the treated-animals decreased over the treatment period, it seemed that the increase in relative organ weight was not directly related to DEHP treatment.
Even though the TP, TG, and Cl levels were unchanged, similar to those of the DEHP-treated group, treatment related effects, including observed changes in hematology (increases in the NEU# and NEUT% and the decrease of the LYMPH%) and the histological changes in the liver and testis, were also shown in the 500 mg/kg DINP-treated group. Other significant changes included elevations in PDW, RBC, HGB, HCT, MCHC, and MONO% and reductions in the RET% and RET#, as well as increases in Glu and decreases in Ca in the 125 mg/kg DINP-treated groups. Allowing that the above-mentioned abnormal changes in the hematology and clinical chemistry parameters did not happen in the middle (250 mg/kg) and high dose (500 mg/kg) groups and no dose-dependent effects were observed, it seemed that these abnormal changes were not related to the adverse effects of DINP. To summarize the current results, it can be assumed from this 4-wk study in rats that the no obvious adverse effect level (NOAEL) was 250 mg/kg, based on the observed pathological changes in the liver and testis, which were associated with increases in NEU# and NEUT% and decreases in LYMPH% at the 500 mg/kg DINP dose.
The chronic and subchronic toxicity of DINP administration by gavage has been investigated systematically [5-8,15]. Although toxicological data were often inconsistent from study to study, the liver and kidney were the known toxic target organs in rats [8,16-20]. With respect to the previous results, liver toxicity at the higher dose primarily consisted of hepatic biochemical changes (increased ALT and AST), an increase in liver weight and histopathological changes [6,17,18,20]. In addition, the increase in kidney weight, changes in physiological parameters (increases in blood urea and/or blood creatinine concentrations), and histological abnormalities were the primary renal effects induced by higher oral doses of DINP [19,20]. However, few studies reported testis toxicity of DINP when given orally in rats, except Seung, et al. [5], who demonstrated that when given orally for 4 wk at a dose of 500 mg/kg in Sprague-Dawley rats, DINP caused the sperm count and motility to decrease, whereas the testis weight changed insignificantly [5].
Different from the toxicological effects induced by oral administration of DINP, the pathological changes in the liver and testis without clinical chemistry abnormalities were the primary treatment related effects caused by iv infusion of DINP, whereas kidney effects were not observed. The different toxicities observed when DINP was intravenously and orally administered may be closely related to its toxicokinetic properties. There was no toxicokinetic information regarding DINP exposure after iv administration. However, pharmacokinetic studies of DINP demonstrated that the pharmacokinetic properties of DINP were similar to those of other high-molecular-weight phthalates [21]. When administered orally, DINP was rapidly metabolized in the gastrointestinal tract and then de-esterified to the monoester, which was further metabolized by side-chain oxidation of the ester group or by hydrolysis to phthalic acid [22]. Exposure to DINP at high doses or repeated administration may increase the formation of oxidation products [23]. Shortly after administration, DINP was found primarily in the liver and kidneys, but did not accumulate in any organ or tissue. Following the toxicokinetic research, the toxicological effects of a series of phthalate diesters, including DINP, and their metabolites were evaluated and compared in vitro and in vivo. The results demonstrated that the adverse effects of the metabolic products, including the monoesters, were greater than those of the phthalate esters (PEs) [5,24]. The explanation of the difference in toxicological effects between i.v. and oral exposure may lead to further toxicokinetic investigation following i.v. DINP administration.
DINP is being developed as an alternative to DEHP for use as a plasticizer. A comparison of the toxicological effects of DEHP and DINP following i.v. administration at the same dose (500 mg/kg) indicated that DEHP was more likely to cause adverse effects than DINP. First, although there was no significant difference between the incidence of pathological changes in the liver and testis, the number of animals with obvious pathological changes in the DEHP-treated group was higher than that in the DINP-treated group. Second, obvious pathological changes in the kidney occurred only in DEHP-treated rats. Third, DINP did not affect any clinical chemistry parameters, whereas DEHP cause a significant increase in TP, Alb, TG, and CK. Last, the productive and behavioral effects of DINP in perinatally exposed rats were less potent than those of DEHP [25].
To the best of our knowledge, this is the first report of the toxicological effects of intravenously administered DINP. Our study given the NOAEL of DINP (250 mg/kg) when it was IV administration and suggested that the toxicology of DINP might be lower than that of DEHP, which might be useful in the risk assessment of DINP and its potential use in medical services.
The authors have no conflicts of interest to declare.
This work was supported by the National Natural Science Foundation of China (No 81402847).