Periprocedural management of aspirin and clopidogrel, antiplatelet agents, for Endobronchial Ultrasound with Transbronchial Needle Aspiration (EBUS-TBNA) remains unclear. Discontinuation of antiplatelet therapy has been questioned given the low risk of hemorrhagic complications reported with this procedure. This study evaluated bleeding events following EBUS-TBNA in patients exposed to Dual Antiplatelet Therapy (DAPT), clopidogrel, or Aspirin (ASA) less than seven days prior to procedure compared to patients unexposed to antiplatelet medications.
Retrospective analysis was conducted on patient's ≥ 18 years who underwent inpatient EBUS-TBNA between 1 January 2009 and 31 December 2014.
406 patients were evaluated: DAPT n = 23; ASA n = 99; clopidogrel n = 13; unexposed n = 271. Bleeding events occurred in 2.5% of patients: DAPT n = 2 (8.7%); ASA n = 0 (0%); clopidogrel n = 1 (7.7%); unexposed n = 7 (2.6%), p = 0.05. Exposed patients were more likely to experience hemoglobin drop > 2 g: DAPT 4.3% Vs. clopidogrel 7.7% Vs. unexposed 0.7%, p = 0.02. Patients taking DAPT were more likely to be readmitted in 48 hours for hemoptysis or anemia: DAPT 4.3% Vs. unexposed 0.4%, p = 0.06.
Patients exposed to DAPT/clopidogrel who undergo EBUS-TBNA are more likely to experience delayed bleeding events within 48 hrs of procedure; however, risk of significant bleeding requiring procedural or transfusion intervention is low. Careful operator assessment of individualized risk versus benefit of antiplatelet therapy continuation must occur prior to EBUS-TBNA procedure.
EBUS-TBNA, Bleeding, Clopidogrel
EBUS: Endobronchial Ultrasound; TBNA: Transbronchial Needle Aspiration; DAPT: Dual Antiplatelet Therapy; ASA: Aspirin
Antiplatelet medications, such as clopidogrel and aspirin, are commonly used in the primary and secondary prevention of cardiovascular disease. Given the risk of hemorrhagic complications related to antiplatelet therapy, various guidelines have been published regarding use in the periprocedural period. Use of clopidogrel alone, and in combination with aspirin, during transbronchial biopsy have been associated with significant and severe bleeding events [1,2], while aspirin alone has not been associated with increased bleeding risk [3]. The British Thoracic Society 2013 consensus guidelines recommended discontinuation of clopidogrel 7 days prior to endobronchial or transbronchial biopsy, while aspirin can be continued [1,4]. However, it remains unclear if recommended guidelines for management of antiplatelet therapy for standard transbronchial sampling can be extrapolated to endobronchial ultrasound with transbronchial needle aspiration (EBUS-TBNA), a newer endobronchial procedure with significantly lower baseline bleeding complications.
EBUS-TBNA is considered minimally invasive and more cost-effective than mediastinoscopy, with superior diagnostic yield compared to transbronchial biopsy and radiographic cancer staging [5]. In the hands of experienced operators, EBUS with TBNA has improved the overall safety, sensitivity, and specificity of obtaining tissue from the mediastinum [6-9]. In a large cohort of more than seven thousand patients undergoing EBUS-TBNA, adverse events occurred in 1.23% of patients, with the most common issue being bleeding (0.68%); however, antiplatelet use was not thoroughly documented in this population [10]. In contrast, standard (non-EBUS) TBNA carries a "moderate" bleeding risk (7.7%), independent of antiplatelet therapy [1,11].
To date, evidence regarding the perioperative management of patients who are exposed to antiplatelet therapy just prior to or during EBUS-TBNA is limited to small case series, case report, and consensus opinion [10-14]. Thus, we sought to further evaluate and define the frequency of adverse bleeding events in patients exposed to either single-agent or Dual Antiplatelet Therapy (DAPT) within seven days of EBUS-TBNA procedure as compared to patients without antiplatelet exposure or discontinuation of therapy more than seven days prior to procedure.
We retrospectively examined records of all adults aged 18 years or older who underwent inpatient EBUS-TBNA at OhioHealth Riverside Methodist Hospital between January 1, 2009 and December 31, 2014. Patients were identified using the following CPT codes: 31620, 31629, 31633. Individuals were excluded from the study if there had been prior diagnosis of bleeding disorder (VonWillebrand's disease, Factor V deficiency); platelet counts less than 50,000; active DIC; or documented clotting disorder (Antithrombin III deficiency, or Antiphospholipid syndrome, Protein S/C deficiency). Additionally, patients taking therapeutic anticoagulants, including warfarin, bivalrudin, heparin, low-molecular-weight heparin, rivaroxaban, argatroban, dabigatran, or fondaparinux, were excluded. Even less is known about antiplatelet medications such as prasugrel, ticagrelor, dipyridamole, cilostazol, or ticlopidine and, as such, they were also excluded from our analysis. Patients receiving DVT prophylaxis-dosed heparin or low-molecular-weight heparin were identified and included in analysis. All EBUS-TBNA procedures were performed using conscious sedation with an Olympus bronchoscope. Both Cook and Olympus EBUS needles were used per operator's preference.
The study was approved by the Ohio Health Institutional Review Board, with approval number OH1-14-00573.
The exposed group was defined as patients taking DAPT (clopidogrel and aspirin), aspirin, or clopidogrel less than or equal to seven days prior to EBUS with TBNA. The unexposed group was defined as patients who took neither clopidogrel nor aspirin, or who discontinued antiplatelet therapy more than seven days prior to procedure.
Adverse bleeding events were defined as any one of the following: immediate bleeding requiring procedural interruption and/or topical intervention (cold saline, epinephrine, tamponade), red blood cell transfusion within 24 hours of procedure, hemoglobin drop greater than or equal to two grams, or readmission within 48 hours of procedure for hemoptysis or anemia.
Data extracted from chart review included adverse bleeding events and comorbid conditions: Chronic Obstructive Pulmonary Disease (COPD), Diabetes (DM), Congestive Heart Failure (CHF), Coronary Artery Disease (CAD)/Myocardial Infarction (MI), End-Stage Renal Disease (ESRD), Cerebrovascular Disease (CVA)/Transient Ischemic Attack (TIA), Hypertension (HTN), cancer, cirrhosis, and Body Mass Index (BMI). We also collected procedural information, including operator, needle size, number of passes, and stations biopsied during the procedure. EBUS-TBNA was performed by five different pulmonologists during this period; no fellows or trainees were involved in the cases.
The data were then merged with an administrative dataset obtained from our hospital support department, which included date of birth, gender, race/ethnicity, payer type, admission date, and discharge date.
Descriptive information on the study sample was tabulated, using means, standard deviations for continuous variables, and percentages for dichotomous/nominal variables. Comparisons of demographic and clinical factors for the cohorts were made using one-way ANOVA tests for continuous data and chi-square tests for dichotomous/nominal data.
Of the 455 patients identified via administrative billing data, 49 were excluded; see Figure 1. Of the remaining patients, the groups exposed to dual and single antiplatelet agents remained small (DAPT n = 23; aspirin n = 99; clopidogrel n = 13; unexposed n = 271) (Figure 1). An average of 5.9 (standard deviation 2.5) passes were completed during lymph node sampling with no difference between cohorts (p = 0.77). Needle gauges ranged from 19 to 25, with median needle size of 22 and no difference noted between cohorts (p = 0.36).
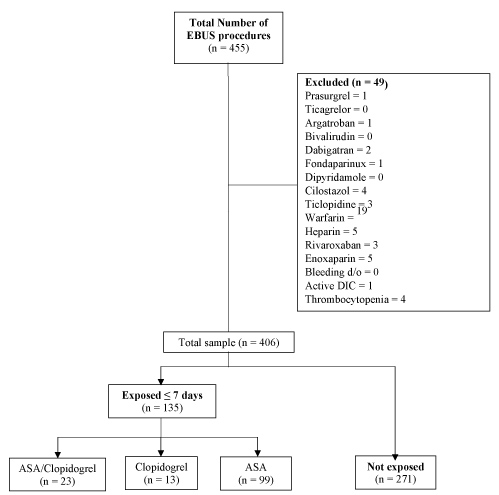 Figure 1: Study flowchart. CONSORT diagram demonstrating flow of participants in the study. EBUS = Endobronchial Ultrasound; TBNA = Transbronchial Needle Aspiration; ASA = Aspirin. View Figure 1
Figure 1: Study flowchart. CONSORT diagram demonstrating flow of participants in the study. EBUS = Endobronchial Ultrasound; TBNA = Transbronchial Needle Aspiration; ASA = Aspirin. View Figure 1
The baseline characteristics were significantly different among the groups (Table 1). The exposed cohort was more likely to be older and have higher rates of COPD, CAD/MI, CVA/TIA, and HTN than were patients in the unexposed group. Patients in the antiplatelet groups were also more likely to be exposed to DVT prophylaxis.
Table 1: Patient characteristics - by exposure group. View Table 1
Overall bleeding events were rare, occurring in 2.5% (n = 10) of all patients (Table 2). No bleeding events required immediate intervention (epinephrine, cold saline, or tamponade) for patients taking DAPT, aspirin alone or clopidogrel alone. However, patients taking DAPT or clopidogrel alone were more likely to experience hemoglobin drop greater than two grams, as compared to their unexposed counterparts (DAPT n = 1 (4.3%); clopidogrel n = 1 (7.7%); unexposed n = 2 (0.7%); p = 0.02). Also, there was a trend of patients exposed to DAPT being more likely to be readmitted within 48 hours of the procedure for hemoptysis or anemia (DAPT n = 1 (4.3%); unexposed n = 1 (0.4%), p = 0.06) (Table 2).
Table 2: Bleeding outcomes - by exposure group. View Table 2
Of patients with a hemoglobin drop greater than two grams, one patient required thoracentesis within 48 hours of EBUS-TBNA; however, pleural fluid analysis revealed a transudative effusion and would not account for hemoglobin drop. No patient required transfusion of blood products.
Our data suggest that patients exposed to antiplatelet therapy may be at risk for adverse bleeding events. No other events or procedures were reported which could account for the change in hemoglobin or need for readmission. Overall, bleeding complications were rare; the absolute number of patients in all groups who experienced bleeding was quite low.
Information on bleeding complications during or following EBUS-TBNA in patients exposed to antiplatelet agents is limited. Three separate reports suggest that EBUS-TBNA can be performed safely by experienced operators in patients taking clopidogrel. Stather and colleagues reported no significant adverse bleeding events in 12 patients who underwent out patient EBUS-TBNA while taking clopidogrel or DAPT at time of procedure or within the 4 week period following the procedure [11]. Significant bleeding was described as > 5 ml of blood loss, interruption of procedure or necessity of intervention with iced saline/epinephrine application.
A published abstract evaluated immediate bleeding complications in 15 patients who were actively taking or had taken clopidogrel within five days of EBUS-TBNA. No significant bleeding events occurred at time of procedure or at 24 hour follow-up and the authors concluded that EBUS-TBNA can safely be performed with a 21- or 22-gauge needle and provide high diagnostic yield in patients taking clopidogrel at the time of the procedure [12].
Finally, Meena and colleagues reviewed 395 consecutive endoscopic procedures, including both EBUS and endoscopic ultrasound via the esophagus with tissue sampling. A total of 17 patients underwent EBUS-TBNA while on clopidogrel, however, information regarding dual antiplatelet therapy was not provided. No clinically significant bleeding complications occurred in those patients taking clopidogrel at time of biopsy or in the control group. The investigators concluded that clinicians can safely proceed with either EBUS-TBNA or EUS-FNA when biopsy results carry important clinical implications and clopidogrel cannot be discontinued [14].
In contrast, a recent case report described moderate bleeding during EBUS-TBNA in a patient taking both aspirin and clopidogrel [13]. The patient had recent placement of a bare metal stent for NSTEMI and was started on appropriate DAPT. After discussion with the patient's cardiologist, the decision was made to continue DAPT during EBUS-TBNA. The patient experienced persistent bleeding, which prolonged the procedure and required tamponade, leading the authors to caution readers about the paucity of evidence regarding EBUS-TBNA and concurrent antiplatelet therapy [13].
Current evidence suggests that premature withdrawal of DAPT may lead to deleterious effects, such as acute coronary syndrome, stroke, and sudden cardiac death [15-17]. Older studies report that the highest risk of thrombotic complications for patients occurs for 4 to 6 weeks after stent placement, though elevated risk persists for at least 1 year following DES placement. However, newer literature suggests increased thrombotic risk is closer to 6 months, irrespective of type of stent and that "newer-generation" DES stents appear to require shorter duration of therapy with DAPT (2016 ACC guidelines).
The 2016 ACC/AHA update recommends that elective noncardiac surgical procedures should be delayed in patients with BMS placement less than 30 days and for patients with DES placement less than 3 months, because of increased harm from discontinuation of DAPT therapy. Further, aspirin should be continued if P2Y12 inhibitor discontinuation is necessary for patients undergoing non cardiac elective surgery [18]. However, these guidelines acknowledge that "the magnitude of incremental bleeding risk in patients treated with antiplatelet therapy who undergo surgery is uncertain" and clinicians should individualize treatment plans based on risks of bleeding versus risk of thrombosis.
Though our study found antiplatelet therapy to be associated with increased delayed bleeding (> 2 gram drop in hemoglobin as measured by CBC, or readmission within 48 hours of procedure), the significance of the > 2 gram drop in hemoglobin can be questioned as there were no differences seen regarding need for immediate intervention, need for transfusion, or length of hospital stay. Only one patient in the DAPT group required readmission as did one patient in the unexposed cohort. Thus, our findings suggest that in the hands of experienced operators, following a discussion of risks versus benefits with the patient, EBUS-TBNA can be safely performed in patients who must remain on DAPT because of high risk of thrombosis if antiplatelet therapy is discontinued. The decision to proceed with EBUS-TBNA should be individualized and include consideration of risk related to procedural delay, as well as risk of bleeding on antiplatelet therapy versus risk of ischemia or thrombosis if medication is stopped.
The implications of our findings are limited by the small numbers of patients and low absolute number of bleeding complications in both exposed and unexposed groups. A larger patient population size is likely required to achieve adequate statistical power and to avoid type II error, given the very low incidence of overall bleeding complications with EBUS-TBNA. Further, our study was retrospective in nature; bronchoscopists were likely aware of patient exposure to antiplatelet agents, and thus may have been more cautious in procedural approach, leading to fewer immediate bleeding-related events. Antiplatelet therapy may have been discontinued prior to the procedure in patients thought to be at high risk from bleeding.
We excluded patients on antiplatelet medications other than clopidogrel given the lack of inclusion in major guidelines during this study period [ACCP, BTS]. This only accounted for one case in this series. As these medications become more commonly used, it will be important to include them in future analyses.
We evaluated only patients admitted to the hospital for the procedure. Outpatient procedural complications were not included in order to monitor both immediate and delayed bleeding events with objective laboratory measurement. Future evaluation of EBUS-related bleeding events may examine outpatient complication rate. A larger study with appropriate blinding would be needed to draw more defined conclusions. Additionally, significant differences in patient characteristics (demographics and comorbid conditions) were noted between exposed and unexposed groups. Thus, it may be that the differences detected in bleeding events could be attributable to the increased average age and rate of cardiovascular comorbid conditions of the exposed group. Again, a larger study would be needed to either statistically control for the differences or to conduct an analysis via propensity score matching.
Our retrospective cohort study offers additional assessment of the risk of adverse bleeding events, comparing inpatients on DAPT, clopidogrel alone, aspirin alone and non-exposed patients. Though EBUS-TBNA is safe and minimally invasive in the hands of experienced operators, our findings suggest that rare late bleeding events may be higher in patients exposed to DAPT or clopidogrel alone within seven days of the procedure. However, given the overall low risk of significant bleeding requiring procedural or transfusion intervention, our findings support continuation of therapy in patients deemed at high risk of thrombosis with short-term withdrawal of antiplatelet therapy. Careful operator assessment of individualized risk versus benefit of antiplatelet therapy continuation must occur prior to EBUS-TBNA procedure.
KS and RG had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis, and especially any adverse effects. JOE contributed to study design, data analysis, creation of Figure 1, creation and revisions of Table 1 and Table 2 and interpretation and final approval of the draft to be published. KS, RG, and KJ contributed substantially to the study design, data analysis and interpretation, writing and revision of the manuscript, revisions of Figure 1, Table 1 and Table 2 and final approval of the draft to be published.
We have no potential conflicts of interest with any companies/organizations whose products or services may be discussed in the article. This project did not have any associated funding.