The correlation between glomerular podocytes and renal tubular cells involved in handling of leaked proteins and the level of proteinuria was evaluated. Retrospective 22 cases of clinical proteinuria in patients with various glomerulopathies were retrieved and analyzed. Glomerulopathies in the concerned patients were pathologically diagnosed through light and electron microscopic examination of the submitted renal biopsies. Three cases with protein levels in urine within the acceptable normal range were additionally analyzed as controls. Electron microscopic examination of the glomerular podocytes and immunofluorescence of the renal tubular epithelium in the relevant cases constituted the base for the present study. Among the studied cases, it was found that the greater the number of glomerular podocytes with reabsorbed intracytoplasmic proteins and the higher score of tubular protein immunofluorescence, the lower the level of proteinuria. Comparatively, cases with fewer number of podocytes with reabsorbed proteins and lower score of tubular protein immunofluorescence had higher levels of proteinuria.
The present study aims to pay the attention to the correlation between morphologically recognizable glomerular podocytes and renal tubular epithelium with reabsorbed proteins and the level of proteinuria in patients with various glomerulopathies. The current study may serve as a base for the future research work concerned with the structural changes of glomerular podocytes and renal tubular epithelium as a compensative mechanism in cases of proteinuria.
Podocytes, Tubular epithelium, Reabsorbed proteins, Proteinuria
Podocytes (glomerular visceral epithelial cells) are highly specialized cells constituting a crucial component of the three-layered glomerular filtration barrier of the kidney [1,2]. Failure of podocyte function is involved in the progression of chronic glomerular disease [3]. Renal tubular cells are actively engaged in reabsorption of proteins that may leak into urine due to disruption of the glomerular filtration barrier [1-7].
Proteinuria is the condition characterized by the presence of greater than normal amounts of proteins in the urine [4-6,8-11]. There are 3 main causes of proteinuria, namely; glomerular diseases, increased quantity of proteins in serum (overflow proteinuria), and low reabsorption of the renal proximal tubules [7,12,13]. In case of glomerular diseases, the glomerular filtration barrier is damaged permitting proteins such as albumin to leak from the blood into the urine. Proteinuria can be classified on the basis of protein amount (nephrotic or non-nephrotic), on the type of protein (albuminuria or low molecular weight proteinuria), or on the underlying pathological changes (glomerular vs. non-glomerular) [14-16]. Low protein reabsorption of the renal proximal tubules is one of the three main causes of proteinuria [7,12]. Renal tubular dysfunction is accordingly classified as a non-glomerular contributing factor in the development of proteinuria. The aim of the present retrospective study is to evaluate the correlation between the glomerular podocytes and renal tubular epithelium containing reabsorbed proteins, as recognizable by transmission electron microscopy and immunofluorescence, and the level of proteinuria in patients with various glomerulopathies.
The study was conducted in Anatomic Pathology Section, Department of Pathology and Laboratory Medicine, College of Medicine, King Saud University, over a period extending from January, 2013 to December, 2015. Twenty-two patients with clinical proteinuria were enrolled in the study with the involvement of additional three patients with protein levels in urine within the acceptable normal range as controls. At the time of sample collection, none of the concerned patients received any treatment that may influence the results of urine samples analysis.
Renal biopsies obtained from all patients were processed for light microscopic examination and all staining protocols, including the routine and immunofluorescence staining, were carried out.
Transmission electron microscopic examination of the renal biopsies was performed to examine the glomerular podocytes at the ultrastructural level. Briefly, renal tissues were primarily fixed in 2.5% buffered glutaraldehyde (phosohate buffer, pH 7.2) and post-fixed in 1% osmium tetroxide (OsO4). Tissues were then dehydrated in ascending series of ethyl alcohol and subsequently embedded in epoxy resin (Epon: Araldite mixture). Semi-thin tissue sections (0.5 µm thickness) were made and accordingly ultra-thin sections (70-85 nm thickness) were prepared and double stained with uranyl acetate and lead citrate. Ultra-thin tissue sections were examined and photographed under a transmission electron microscope (TEM) (JEM-1400 TEM, JEOL Co., Tokyo, Japan) operating at 100 kV.
The appropriate methods, including Weibel and Gomez point counting method, described in previous studies [9,17,18] to measure the proportion of glomerular cell types were employed. Accordingly, the glomerular podocytes were counted and the average number of podocytes/glomerulus in each of the examined cases was calculated. The average number of podocytes containing reabsorbed intracytoplasmic proteins/glomerulus was also estimated.
Reabsorbed proteins (albumin) in renal tubular cells were the target immunofluorescence (IF) staining. Serial frozen sections were prepared from the unfixed renal tissues and treated with a fluorescein-labelled antibody specific for albumin. The intensity of IF staining in renal tubular cells was expressed semi-quantitatively as: negative, no reabsorption proteins; mild, less than 25% of the tubules show reabsorption proteins; moderate, 26% to 50% of the tubules show reabsorption proteins; and severe, more than 50% of the tubules show reabsorption proteins. The corresponding reabsorbed albumin scores as observed by light microscopy in renal tubular cells were: 0, no significant hyaline cytoplasmic reabsorption droplet change; 1+, Minimal hyaline reabsorption droplet change; 2+, Mild hyaline reabsorption droplet change; 3+, Moderate hyaline reabsorption droplet change.
Data on counting of glomerular podocytes with reabsorbed proteins, immunofluorescence scoring of reabsorbed proteins and albumin scores in the renal tubular cells, and proteinuria levels were recorded and presented as mean and standard deviation. Statistical analysis of the data was done using Wilcoxon Mann-Whitney U test (p-value = 0.035).
Immunofluorescence (IF), electron microscopic findings, and the pathological diagnosis for each of the studied 25 cases was summarized in (Table 1A and Table 1B). Because of the existing variations of disease etiology, the studied cases were divided into 2 groups; the first group (Table 1A) encompassed those cases with immunocomplex deposition-related glomerulonephritis, and the second one involved those with non-immunocomplex-related glomerulopathies.
Table 1A: Immunoflourescence, electron microscopic findings, and pathological diagnosis in 25 patients with various glomerulopathies. A. Immunocomplex deposition-related glomerulonephritis. View Table 1A
Table 1B: Immunoflourescence, electron microscopic findings, and pathological diagnosis in 25 patients with various glomerulopathies. B. Non-immunocomplex-related glomerulopathies. View Table 1B
In (Table 2), demonstrate the level of proteinuria, average number of podocytes/glomerulus, and average number of podocytes with reabsorbed intracytoplasmic proteins/glomerulus in 25 patients with various glomerulopathies. The average number of podocytes/glomerulus was variable among the studied disease entities and ranged from 20-26. The average number of podocytes with reabsorbed intracytoplasmic proteins/glomerulus ranged from 0-5. The highest average number of podocytes with distinct reabsorbed proteins/glomerulus (3-5 cells/glomerulus) were found in cases with low proteinuria levels (0.49-2.32 gm/day). Cases with the average number of only one podocyte with reabsorbed proteins/glomerulus revealed the highest level of proteinuria ranging from 4+ to 28.97 gm/day.
Table 2: Level of proteinuria, average number of podocytes/glomerulus, and average number of podocytes with reabsorbed intracytoplasmic proteins/glomerulus in 25 patients with various glomerulopathies. View Table 2
Contents of (Table 3) clearly indicated that cases in high number group (three-five podocytes with reabsorbed proteins/glomerulus) manifested the lowest level of proteinuria, while the highest level of proteinuria was encountered in cases of low number group (one podocyte/glomerulus).
Table 3: Groups of cases with various numbers of glomerular podocytes with reabsorbed intracytoplasmic proteins/glomerulus; Three to five podocytes (high), Two podocytes (moderate), One podocyte (low), and the average level of proteinuria in each group. View Table 3
Levels of proteinuria, tubular immunofluorescence and albumin scores of reabsorbed proteins in the renal tubular epithelium as well as tubular cell injury in each case were shown in (Table 4).
Table 4: Albumin and immunofluorescence scores of tubular reabsorbed proteins, tubular cell injury and level of proteinuria in patients with various glomerulopathies. View Table 4
Figure 1 and Figure 2 show the immunofluorescence (IF) staining in two different cases; a case with mild IF, less than 25% of the tubules showing reabsorption proteins (Figure 1), and another case with severe IF, more than 50% of the tubules show reabsorption proteins (Figure 2).
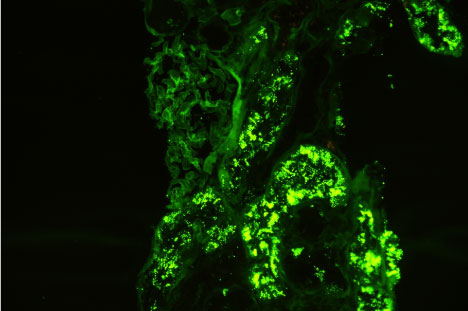 Figure 1: Immunofluorescence micrograph of a renal tissue showing severe IF staining, more than 50% of the tubules show positivity for reabsorbed albumin in their lining epithelium. View Figure 1
Figure 1: Immunofluorescence micrograph of a renal tissue showing severe IF staining, more than 50% of the tubules show positivity for reabsorbed albumin in their lining epithelium. View Figure 1
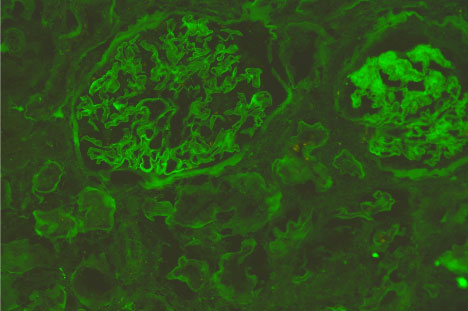 Figure 2: Immunofluorescence micrograph of a renal tissue show negative tubular IF staining for reabsorbed albumin. View Figure 2
Figure 2: Immunofluorescence micrograph of a renal tissue show negative tubular IF staining for reabsorbed albumin. View Figure 2
Transmission electron microscopy revealed the presence of glomerular podocytes with low and high-reabsorbed intracytoplasmic protein droplets in the examined cases (Figure 3 and Figure 4 respectively). The reabsorbed proteins were recognized as highly electron dense amorphous structures. Prevalence of the reabsorbed proteins in podocytes varied among the different cases and also varied from area to another within the same glomerulus. Podocytes containing cytoplasmic reabsorbed protein droplets were enlarged and disclosed disrupted cytoplasmic organelles and deteriorated surface microvilli.
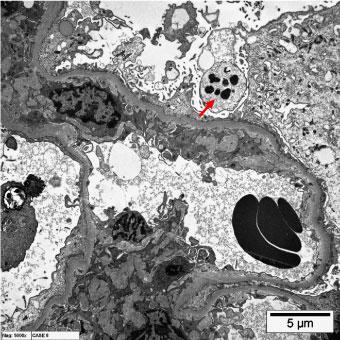 Figure 3: Transmission electron micrographs showing glomerular podocytes with low reabsorbed intracytoplasmic proteins (arrows). (uranyl acetate, lead citrate, x6000). View Figure 3
Figure 3: Transmission electron micrographs showing glomerular podocytes with low reabsorbed intracytoplasmic proteins (arrows). (uranyl acetate, lead citrate, x6000). View Figure 3
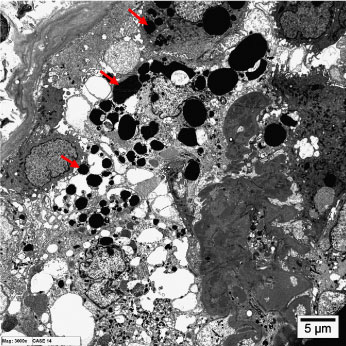 Figure 4: Transmission electron micrographs showing glomerular podocytes with high reabsorbed intracytoplasmic proteins (arrows). (uranyl acetate, lead citrate, x6000). View Figure 4
Figure 4: Transmission electron micrographs showing glomerular podocytes with high reabsorbed intracytoplasmic proteins (arrows). (uranyl acetate, lead citrate, x6000). View Figure 4
Table 5 summarizes the statistical analysis of the obtained glomerular data. Low and moderate groups indicated in Table 3 were merged in one group to be statistically analyzed against the high group. The analysis approved significant differences between the studied groups. No significant differences were detected among cases of different sex and age.
Table 5: Statistical analysis of data relevant to the count of podocytes with reabsorbed intracytoplasmic proteins in the different groups of cases in relation to the level of proteinuria in each group. View Table 5
Statistical analysis of the renal tubular data is shown in a boxplot (Figure 5). A significant negative correlation between the Immunofluorescence tubular scores and level of proteinuria was detected by the statistical analysis.
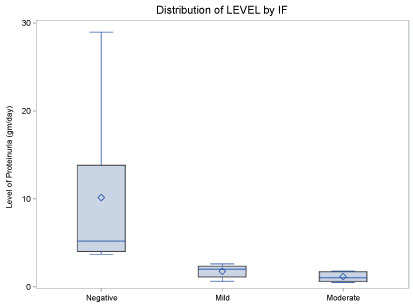 Figure 5: Statistical boxplot showing negative correlation between IF score and various levels of proteinuria. View Figure 5
Figure 5: Statistical boxplot showing negative correlation between IF score and various levels of proteinuria. View Figure 5
Presently, the correlation between the morphologically abnormal podocytes and renal tubular epithelium containing reabsorbed intracytoplasmic proteins and the level of proteinuria was evaluated.
Normal urinary protein excretion is < 150 mg/24 hour, and daily albumin excretion in a normal person is < 30 mg. 11 Loss of proteins in urine is the hallmark of tubular and glomerular diseases, and may arise from structural and/or functional alterations involving different cell types [19-21]. Various proteinuric diseases may share similar renal pathological changes and may have a common progression of renal injury [6,22-25]. Albuminuria is strongly associated with the progression of renal disease [26-28].
Proteinuria of the presently studied cases was most likely of glomerular origin, i.e., at the glomerular level, based upon the encountered glomerular structural changes. Beside the well-known protein endocytosis at the renal proximal tubular cells, there is an increasing evidence of glomerular protein handling by podocytes [22,29-31]. It has been concluded that identifying the mechanisms involved in albumin handling in podocytes is essential to understand the pathogenesis of various glomerulopathies [19]. Such mechanisms in human podocytes are committed to internalizing albumin (albumin endocytosis) through a receptor-mediated mechanism [29].
The structural integrity of podocytes is crucial to guard against leakage of proteins in urine. In this regard, podocytopathies are the most common group of glomerular disorders leading to proteinuria [1,19]. The final clinical scoring of proteinuria is largely related to the podocytes integrity and activity in reabsorbing leaked proteins.
The present results clearly indicate that cases of glomerulopathies with the higher average number of podocytes containing reabsorbed cytoplasmic protein droplets had the lower levels of proteinuria. Subsequently, it can be concluded that the larger the number of morphologically recognizable podocytes containing reabsorbed proteins, the lower the level of the expected clinical proteinuria.
Hyper-filtration of proteins is known to be followed by increased reabsorption in the renal proximal tubules [12]. The basic defects leading to tubular proteinuria arise from proximal tubules, with the result of excretion of proteins that are normally reabsorbed efficiently by the proximal tubular cells through a receptor-mediated endocytosis [6].
The reabsorbed proteins can be cytotoxic to proximal tubular epithelium and extensive reabsorption of large quantity of abnormally filtered proteins may provoke tubular damage [17,24].
Similar to glomerular Podocytes, active reabsorption of filtered proteins by proximal tubular cells is done by endocytosis by the crucial aid of endocytic surface receptors, such as megalin and cubilin as a form of receptor-mediated process [1,5,12]. Changes in the expression and/or subcellular distribution of these two endocytic receptors are expected to be associated with proteinuria due to receptor dysfunction [32,33].
The present data, regardless the type of the underlying glomerulopathies, showed that the greatest average number of podocytes with distinct reabsorbed proteins and the highest immunofluorescence tubular score were detected in cases with lower proteinuria levels. In contrast, cases with the highest proteinuria level had the fewer number of podocytes containing reabsorbed proteins and the lowest IF tubular score. Subsequently, the level of proteinuria and the progression of a proteinuric disease can be predicted through estimation of these parameters.
These findings might indicate a parallel and synergic glomerular and tubular roles to handle, retain and preserve proteins and thus hindering protein leakage into urine and reabsorb proteins that may leak into the glomerular filtrate.
The present work was concentrated objectively on the correlation between the morphologically recognizable reabsorbed proteins in glomerular podocytes and renal tubular epithelium, and the level of clinical proteinuria in patients with glomerulopathies. The current study may hopefully constitute a base for the next investigations concerned with the structural changes of glomerular podocytes and renal tubular epithelium as a compensative mechanism in cases of proteinuria.
However, further investigations on a larger scale of patients with clinical proteinuria are needed to strongly establish the currently evaluated correlation.
The author would like to thank Dr. Mohammed Mubarak for his efforts in the Electron Microscopy technical support (King Saud University) and Dr. Albara Marwa for statistical analysis (King Saud University).
I have no relevant interests to disclose. This research is not funded by any agency.