Aim of the study: In critically ill patients, induced hypothermia or active temperature control to avoid fever are used for neuroprotection. The aim of this study was to determine the feasibility and performance of a novel temperature management system in patients after cardiac arrest, with neurological fever or due to COVID-19 infection.
Methods: In this retrospective observational study, patients admitted to intensive care with different indication for temperature management(induced hypothermia or fever control), such as after cardiac arrest or neurogenic fever (> 37.5 °C), were treated at different target temperatures using a novel system for temperature management (Brain Cool/IQool™) which is a non-invasive and advanced system for temperature management. Target and maintain temperatures were determined for every patient individually, based on patient characteristics indication and local guidelines. Temperature data were recorded from the bladder catheter every 10 seconds during study procedures and stored automatically by the system. Performance endpoints were: Time to reach target temperature; time within target temperature 0.5 C during maintenance phase; and how well the system could control the rewarming rate.
Results: In total, 92 patients were included of which 39 were treated after cardiac arrest at target temperature of 33 °C (n = 22) or 36 °C (n = 17); 43 patients had neurogenic fever and were treated at target temperature between 36-37 °C; 10 patients had severe COVID-19 with refractory fever and were treated at target temperature of 37 °C. Cardiac arrest patients reached target temperature after 2.8 ± 1.3 h, with a mean cooling rate of 1.1 ± 0.4 °C/h. The stability of target temperature within ± 0.5 °C during maintenance phase were 80.2% and 78.8% for patients treated at 33 °C and 36 °C, respectively. During rewarming, patient temperature was increased to 37 °C at rate of 0.2 ± 0.1 °C/h. In neurogenic fever patients, the time to reach target temperature of 36-37 °C was 2.3 ± 0.8 h with a mean cooling rate of 0.8 ± 0.3 °C/h. The overall time at target ± 0.5 °C was 81.1 ± 19.4%. In COVID-19 patients, target temperature of 37 °C was reached after 3.1 ± 1.6 h, with a mean cooling rate of 1.0 ± 0.4 °C/h. The overall time at target ± 0.5 °C was 81.3 ± 29.4%. Besides minor reversible indentation points on the skin, no adverse events were recorded.
Conclusions: In this observational study, the studied novel temperature management system could be used with high performance to reach and maintain target temperature and rewarming in critical ill patients after cardiac arrest, neurogenic fever and refractory fever associated with critical Covid-19 without significant side effects or safety issues.
Cardiac arrest, Targeted temperature management, Temperature, Neurogenic fever, COVID-19, Fever management
Targeted temperature management (TTM) is used as an intensive care unit (ICU) treatment in several critical conditions. In cardiac arrest patients, the results from randomized trials are diverging considerably. While some studies show improvement in survival with good neurologic outcome from TTM with a core body temperature of 33 °C for 24 hours [1,2], other studies show no benefit in survival or neurologic function from other temperature levels, including using only normothermia for 72 hours but with an active control of fever (i.e., avoid core body temperatures > 37.7 °C) [3,4]. However, to avoid fever for 3 days in this setting an active temperature control device is needed in most patients [3].
In addition to the use of TTM in cardiac arrest, fever is common in critically ill patients and has shown to have a negative impact on outcome for many disease types [5,6]. The impact of fever not only results from increased metabolic demand, but also from multiple cellular and molecular effects that cause severe inflammation and injury. Patient temperature control and reduction of fever burden is therefore a major objective of intensive care clinicians. Thus, regardless of target temperature, there is a need for effective and accurate temperature control for several patient groups in the ICU. Currently, there are surface and invasive cooling technologies commercially available [7,8]. However, data on the efficacy of these systems are limited. Precision of temperature control may become even more important if a clinician decides to switch between different target temperatures with a more tailored and patient centered approach.
In this study we retrospectively analyzed patients who were treated after cardiac arrest, patients with neurogenic fever, and patients with COVID-19 that had refractory fever at the ICU. The aim of this study was to evaluate the efficacy and precision of the cooling system where the end points were to measure the time to reach target temperature; to calculate time within target temperature ± 0.5 °C during maintenance phase; and how tightly the system can control the rewarming rate.
Patients admitted to the ICU with conditions requiring TTM such as out-of-hospital cardiac arrest, refractory fever in neurologic conditions (neurogenic fever) and in COVID-19 patients with refractory fever and with need for active temperature control to reduce metabolic demands and neuroprotection.
This study was conducted as a retrospective, observational study including patients admitted to the ICU with indication for TTM at centers in Sweden, USA, Vietnam and South Korea between January 2020 and August 2021. The decision to treat patients with the Brain Cool™/IQool™ system for TTM was made by clinicians according to existing local guidelines. Patients were included retrospectively based on selected clinicians at the participating centers and no randomization took place. There were no control group as patients received standard care and was only included if they were assigned TTM according to local guidelines. Ethical approval for the study was obtained from the Swedish Ethical Review Authority before starting the analysis with the identification number 2022-00264-01.
The Brain Cool™/I Qool™ System consists of a temperature control unit, patient temperature sensors and three separate zone for temperature control: head/neck, torso and thigh. The schematic of Temperature management system is shown in Figure 1. This an advanced surface cooling system with three separate zones. The head/neck cap is intended to induce a localized cranial hypothermic state, thus gaining the potential neurological benefits of hypothermia. It produces high flow rates and provides intimate skin contact with the patient, with the aim to maximize heat transfer. The pads may be pre-filled which enables instant application and allowing therapy to begin in shortly after arrival at the ICU. The Temperature management system has an algorithm with an internal feedback loop that will enables tighter control of patient temperature. The system has an easy-to-use intuitive interface and informative graphics. Body temperature is measured continuously using an esophageal or a bladder temperature probe attached to the system and automatically logging the data. The system is design to be used by ICU nurses or doctors.
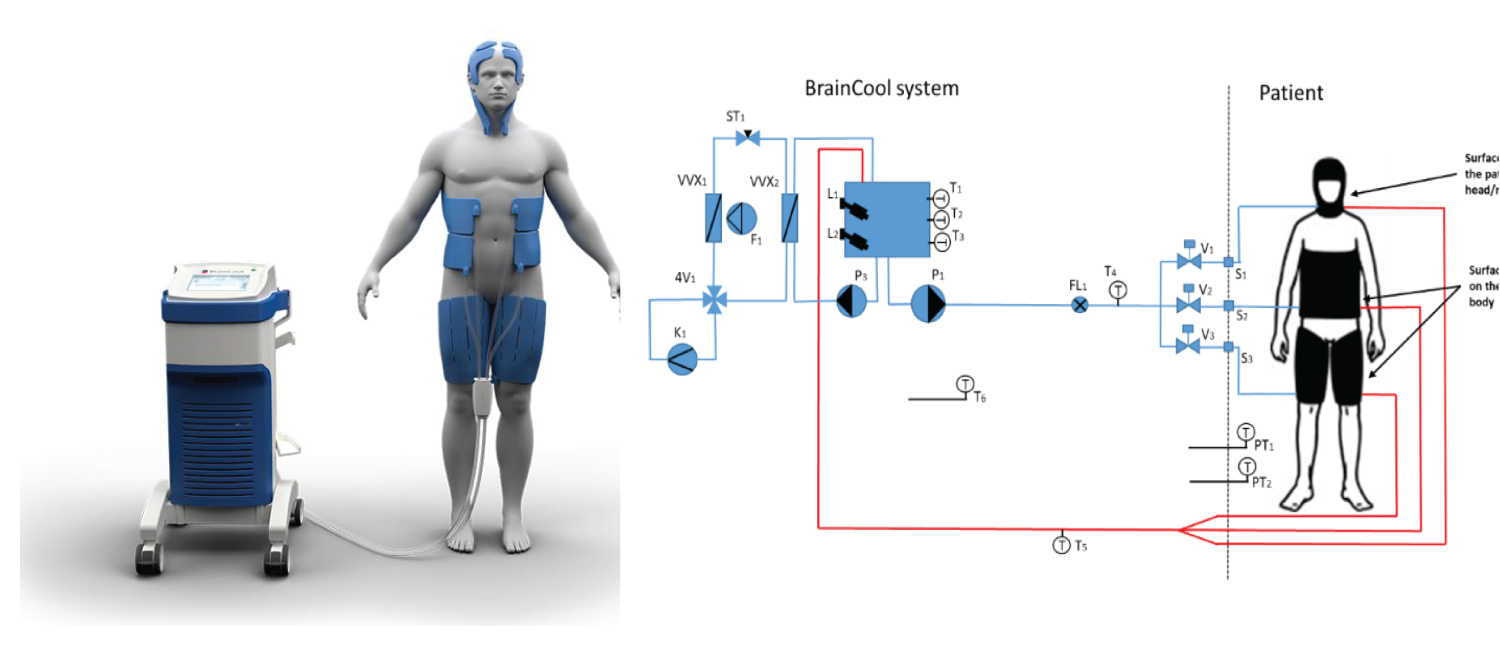 Figure 1: Schematic diagram of the operation of a Brain Cool™/I Qool™ System.
View Figure 1
Figure 1: Schematic diagram of the operation of a Brain Cool™/I Qool™ System.
View Figure 1
Data was collected from clinics in Sweden, USA, Vietnam, and South Korea where patients had been treated in the Medical ICU, general ICU and Neuro ICU with TTM using Brain Cool™/I Qool™ system between January 2020 and August 2021. Data on the indication for temperature management and temperature data was retrieved through the built-in logging system via USB stick memory. No individual data was collected besides temperature data, thus no information can be traced back to individual patients. The data processing and storage are performed in accordance with the provisions of the General Data Protection Regulation (EU ordinance 2016/679, GDPR).
In a single log file, each data point is an average value of 60 recorded points (the system records patient temperature every 10 seconds). In the temperature responses graphs, all data are presented as mean ± standard deviation (SD) unless otherwise noted (Microsoft Excel for Microsoft 365 MSO). In the comparison table, the cooling rate is calculated when the starting temperature of the patient is higher 1 °C than the setting target temperature. In the same way, the rewarming rate is calculated when the rewarming target is 1 °C higher than the patient temperature.
A total of 92 patient were included of which 39 patients were included after CA and treated with TTM at 33 °C or 36 °C, 43 febrile (> 37.5 °C) patients were included from the Neuro ICU, where they were treated to target temperature ranged between 36-37 °C and 10 patients suffering from COVID-19 were included for fever management and treated at target temperature 37 °C.
Table 1 displays a summary of the characteristics of cooling for different indications. Cooling speed was highest in cardiac arrest patients (1.1 ± 0.4 °C/h) and similar in COVID-19 patient (1.0 ± 0.4 °C). For fever management in neurogenic patients, the cooling rate was 0.8 ± 0.3 °C. Time to target temperature was shortest in patients with neurogenic fever (138 min).
Table 1: Characteristics of cooling intervention. View Table 1
Thirty-nine CA patients were enrolled in this analysis. Mild hypothermia at 33 °C was targeted in seventeen patients and the other twenty-two patients were treated at 36 °C. The average time to reach target temperature at 33 °C was 186 min ± 96 min, which corresponds to a cooling rate of 1.1 °C/h ± 0.4 °C/h. The stability of target temperature within ± 0.5 °C during maintenance phase were 80.2% ± 25.3% and 78.8% ± 24.8% for patients treated at 33 °C and 36 °C, respectively. Following maintenance phase, patient temperature was increased to 37 °C at rate of 0.2 °C/h ± 0.1 °C/h during re-warming phase (Figure 2 and Figure 3).
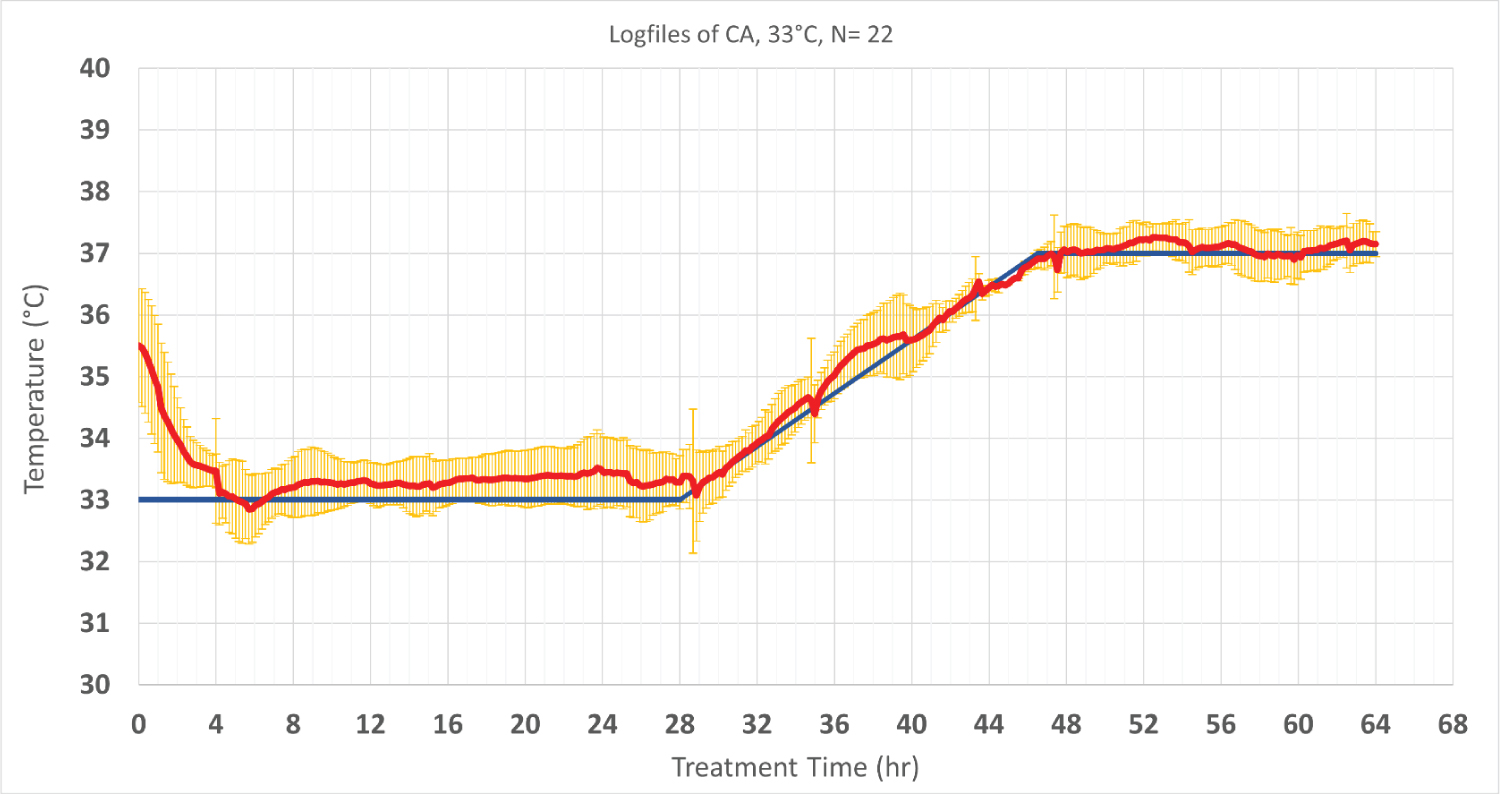 Figure 2: Patients’ temperature and target temperature (hypothermia at 33 °C and normothermia at 37 °C). The data is performed of 22 cardiac patients who were treated in the same setting and period).Values are shown as mean ± SD. The red line shows the patient temperature, and the blue line represents the targeted temperature. Standard deviations are shown in yellow.
View Figure 2
Figure 2: Patients’ temperature and target temperature (hypothermia at 33 °C and normothermia at 37 °C). The data is performed of 22 cardiac patients who were treated in the same setting and period).Values are shown as mean ± SD. The red line shows the patient temperature, and the blue line represents the targeted temperature. Standard deviations are shown in yellow.
View Figure 2
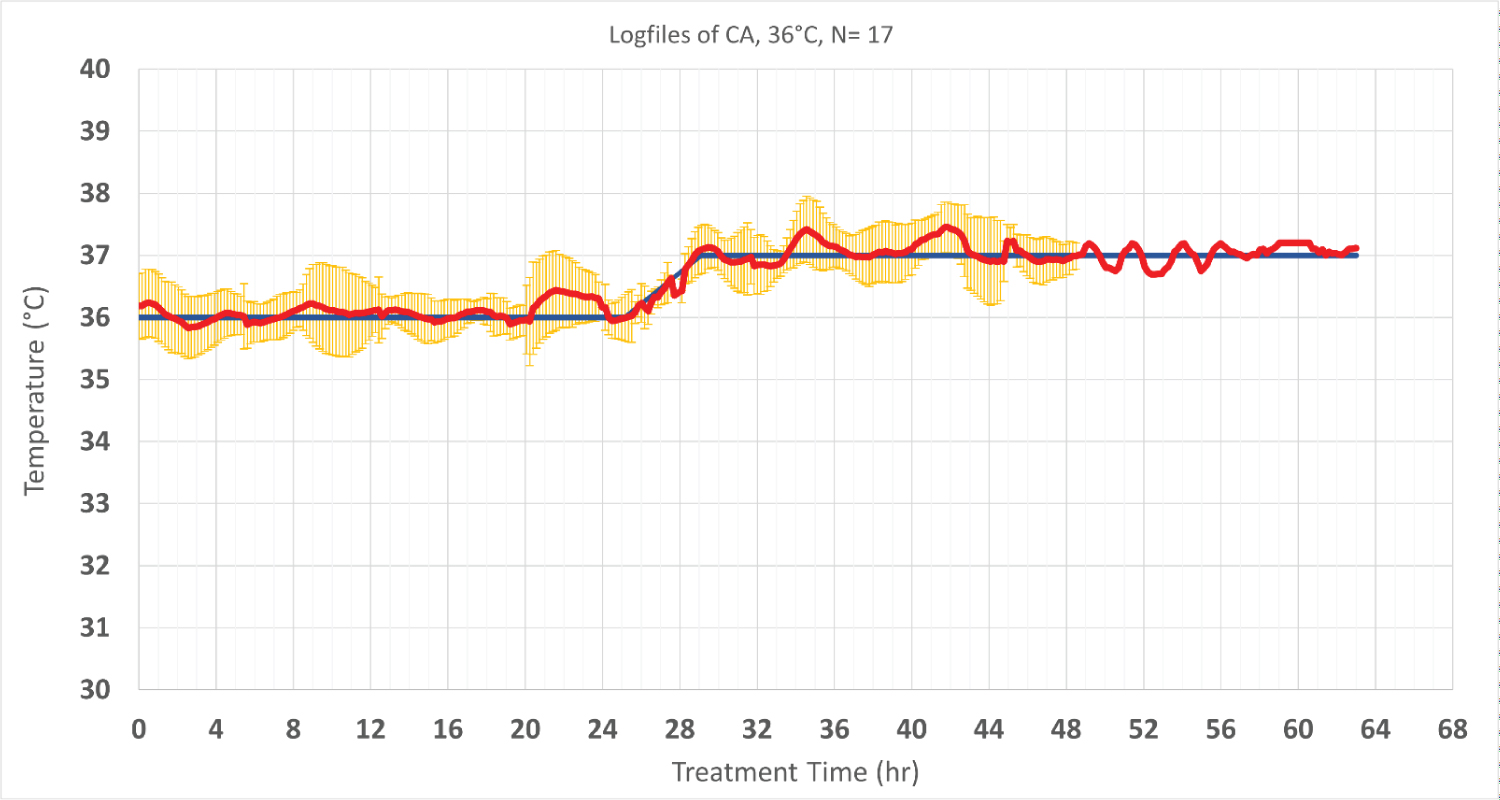 Figure 3: Patients’ temperature and target temperature (hypothermia at 36 °C and normothermia at 37 °C). The data is performed of 17 cardiac patients who were treated in the same setting and period except one patient that data was collected for longer period up to 63 hours).Values are shown as mean ± SD. The red line shows the patient temperature, and the blue line represents the targeted temperature. Standard deviations are shown in yellow.’
View Figure 3
Figure 3: Patients’ temperature and target temperature (hypothermia at 36 °C and normothermia at 37 °C). The data is performed of 17 cardiac patients who were treated in the same setting and period except one patient that data was collected for longer period up to 63 hours).Values are shown as mean ± SD. The red line shows the patient temperature, and the blue line represents the targeted temperature. Standard deviations are shown in yellow.’
View Figure 3
Forty-three patients in the Neuro ICU who were febrile (> 37.5 °C) were treated for fever management. Target temperatures were determined for every patient individually, based on patient characteristics and indication. The treatment targets ranged between 36 °C and 37 °C (Figure 4). In neurogenic fever, the time from beginning of cooling to reaching target temperature was 138 min ± 48 min, resulting in a mean cooling rate of 0.8 °C/h ± 0.33 °C/h. The overall time on target ± 0.5 °C was 83.3% ± 19.4%.
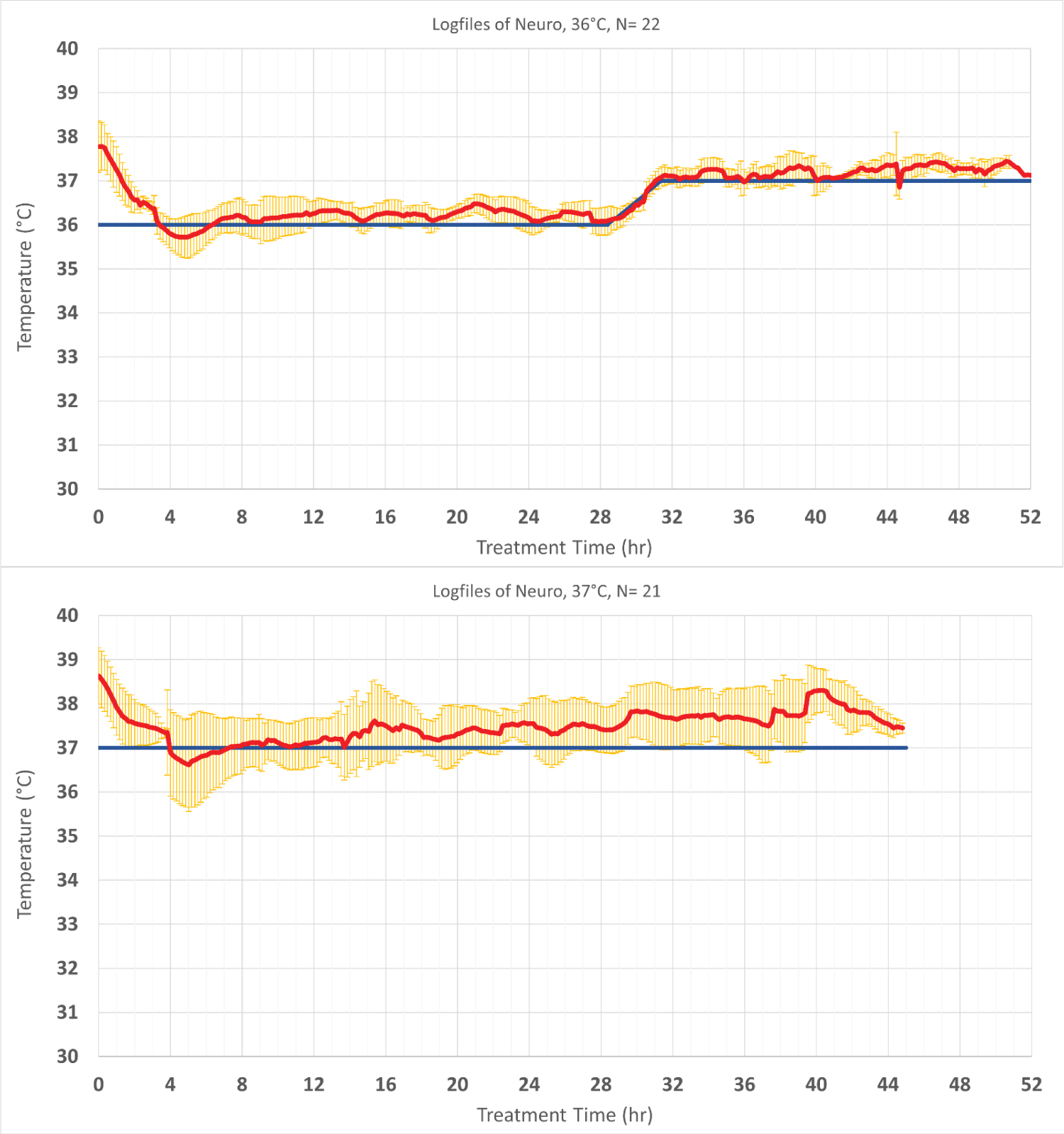 Figure 4: Temperature profile of patients treated to maintain normothermia that was defined < 37.8 °C in the TTM2 study. Values are shown as mean ± SD. The red line shows the patient temperature, and the blue line represents the targeted temperature. Standard deviations are shown in yellow.
View Figure 4
Figure 4: Temperature profile of patients treated to maintain normothermia that was defined < 37.8 °C in the TTM2 study. Values are shown as mean ± SD. The red line shows the patient temperature, and the blue line represents the targeted temperature. Standard deviations are shown in yellow.
View Figure 4
Ten COVID-19 patients that require ICU treatment were treated at 37 °C for fever management. In these patients, the time from beginning of cooling to reach target temperature was 186 min ± 96 min, resulting in a mean cooling rate of 1.0 °C/h ± 0.4 °C/h. The overall time on target ± 0.5 °C was 81.3% ± 29.7% (Figure 5).
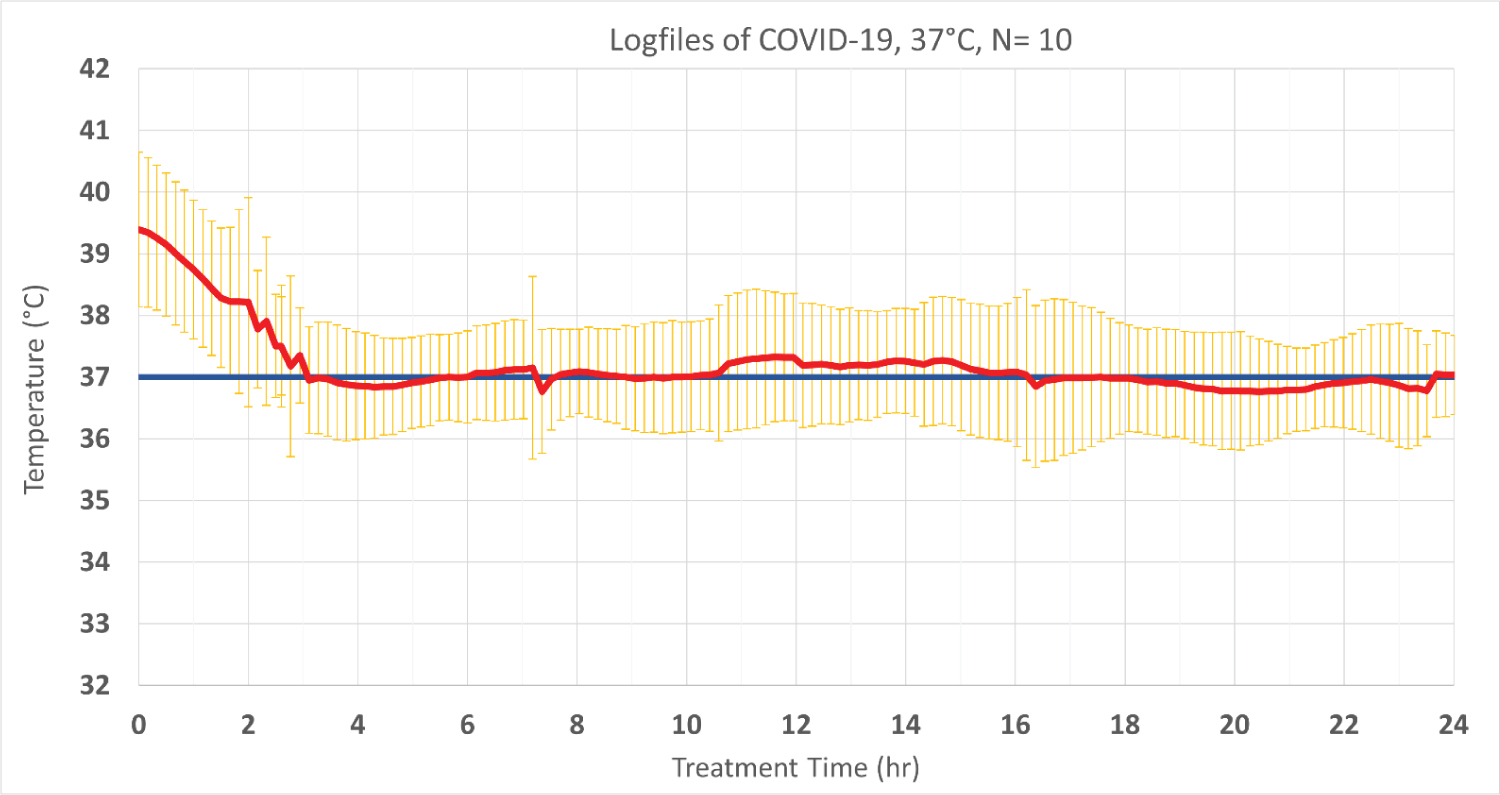 Figure 5: The performance of COVID-19 patient temperature treated by the Brain Cool/I Qool system. Values are shown as mean ± SD. The red line shows the patient temperature, and the blue line represents the targeted temperature. Standard deviations are shown in yellow.
View Figure 5
Figure 5: The performance of COVID-19 patient temperature treated by the Brain Cool/I Qool system. Values are shown as mean ± SD. The red line shows the patient temperature, and the blue line represents the targeted temperature. Standard deviations are shown in yellow.
View Figure 5
There were two reports on minor indentation points on the skin; however, they were self-corrected and did not interfere with treatment. No ulcerations were reported. There were no adverse events linked to the use of the device in any patients.
The overall result of this study showed that the studied novel system for temperature management (Brain Cool™/I Qool™) could reach core body target temperature in average within 2.3-3.1 hours and with adequate quality maintained target temperature appropriate to treat critically ill patients with different indications for temperature control.
The results in cardiac arrest patients demonstrated cooling rates of 1.1 ± 0.38 °C/h during induction phase and provided temperature control during maintenance and rewarming phases. The individual cooling rate in this study ranged from 0.3 °C/h to 1.9 °C/h. The large standard deviation of the average cooling rate might be explained by the patient’s age, shape, size, weight and/or other comorbidities. In addition, cooling was occasionally interrupted due to transport or diagnostic procedures, which may have affected the overall time to target in some patients. The temperature management system was able to automatically control patient’s temperature via temperature feedback to avoid significant overshoot or undershoot of the target temperature. Patient temperature was maintained within ± 0.5 °C of target temperature at 80.2% ± 25.3% and 78.8% ± 24.8% at target temperatures of 33 °C and 36 °C, respectively.
Following TTM-1 trial, some hospitals adopted 36 °C as target temperature instead of 33 °C, however, follow-up observational studies reported low compliance with target temperature at 36 °C, higher rates of fever and a trend towards worse patient outcomes and even increased mortality (survival 71% vs. 58%, p = 0.31, discharge home 58% vs. 40%, p = 0.08, favourable neurologic outcome 71% vs. 56%, p = 0.22) [9]. Allpatients with cardiac arrests are not equal, the survival varies greatly depending on initial rhythm, duration of cardiopulmonary resuscitation and co-morbidities. A recent trial, HYPERION [10], showed that among patients with coma who had been resuscitated from cardiac arrest with non-shockable rhythm, moderate therapeutic hypothermia at 33 °C compared with targeted normothermia at 37 °C led to a significantly higher percentage of patients who survived with a favorable neurologic outcome at day 90. Another crucial issue is the potential benefit of early cooling initiated during cardiopulmonary resuscitation. Studies have shown that the severity of neuronal lesions is dependent on the delay in initiation of cooling after reperfusion [11]. Recent clinical evidence from PRINCESS trial showed that the intra-arrest therapeutic hypothermia, initiated in the field with trans nasal evaporative cooling, compared to standard care could provide benefits in survival with good and complete neurologic outcome in patients with out-of-hospital cardiac arrest with shockable rhythms, although it was not statistically significant for the whole study population [12,13]. The AHA Guidelines is considered the gold standard regarding clinical recommendations in the practice of resuscitation science around the world. According to the recommendations, TTM remains important and prompt initiation is necessary for all comatose patients after return of spontaneous circulation to ensure optimal functional and neurological outcome [14].
Fever is reported in over 40% of stroke patients [15] and although its’ causes are unclear, fever is associated with higher mortality, poor neurological outcomes, increased cranial pressure and prolonged stay in the ICU [16,17]. Studies have shown that control of fever during the acute stages of brain injury is associated with improved outcomes [18,19]. In this study, 43 neurointensive care patients were treated with the Brain Cool™/I Qool™ cooling system for fever management. Patient temperature was maintained within ± 0.5 °C of target temperature at 83.3 ± 19.4 at set temperature of 36 °C. The system was safe to use in these patients and demonstrates a appropriate cooling rate and provide high quality temperature control during maintenance and rewarming phases.
It is becoming increasingly clear that COVID-19 is not merely a respiratory disease, but also may cause brain damage [20]. Blood-clot complications are part of an inflammatory state which increases the risk of stroke and brain damage with subsequent significant needs for rehabilitation. Furthermore, persistent fever in patients is associated with the severity of disease and can worsen gas exchanges in ARDS patients which also enhances brain damage. For these patients, targeted cooling may play an important role to reduce brain damage, improve fever management and respiratory functions by reducing oxygen demand and mitigate neurological complications. In this study, the main objective was to evaluate the feasibility and safety of The Brain Cool™/I Qool™ System for management of severe fever in the COVID-19 patients. It is concluded that the method is safe to use, and it provides appropriate performance regarding cooling rate and temperature control in the maintenance phase. Further studies are needed to evaluate the impact of temperature management in these patients. That may lead to improvement in the treatment practice and outcome of COVID-19 patients in the ICU where high fever has been shown to be deleterious.
In this observational study, the studied novel temperature management system could be used with high performance to reach and maintain target temperature and rewarming in critical ill patients after cardiac arrest, neurogenic fever and refractory fever associated with critical Covid-19 without significant side effects or safety issues.
Funding for the study was provided by the Swedish funding agency (Vinnova).
Mohammad Fazel Bakhsheshi is a full-time Brain Cool AB employee as scientific director. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.