Ab initio calculations were carried out to analyze the interaction between one molecule of B2H4 with H2O, CH3OH, NH3, NH2CH3, NH(CH3)2 and N(CH3)3 molecules at the MP2/aug-cc-pVDZ computational level. B2H4 could act as a hydrogen bond donor through its bridged hydrogens (Hb); while, the B-B can act as a hydrogen bond acceptor. Thus, the interaction of B2H4 with the mentioned molecules resulted in formation of Hb...X and/or B-B...H hydrogen bond complexes; whereas, Ht atoms of B2H4 were ineffective to form Ht...H dihydrogen bond complexes with the amine molecules. Results showed that the B-B...H interactions were stronger than Hb...X counterparts. The obtained structures were analyzed by the natural bond orbital (NBO) and Atoms in Molecules (AIMs) methodologies.
Hydrogen bond complexes, Borane, Amine, B2H4, AIM, MP2
Borane complexes are extensively studied and have even been the subject of Nobel Prize by Brown [1]. Many scientific data exist that have shown that boron is an essential microelement in animal cells. With the knowledge that borate linkages function in cell-to-cell adhesion, it has been hypothesised that boronates target structural glycoproteins located along the cytoskeletonplasma membrane-cell wall assembly. On the other hand, boron-carrier molecules can be used as a therapeutic mean to fight cancers [2,3]. Also, they have been the subject of proton affinity experiments in chemical ionization mass spectrometry. Among non-covalent interactions which have been known in boron chemistry, both dihydrogen and hydrogen bonding patterns are particularly significant [4-9].
B2H4, designated as diborane (4), is probably the best known electron-deficient analogue of ethylene [10-13]. B2H4 bears 10 valence electrons for chemical bonding. There are two standard two electron terminal B-H bonds, thus accounting for a total of four electrons. This leaves a total of six electrons to share between the two bridging H and the two B atoms. Consequently, there are two 3c-2e curved 'banana’ B-H-B bridging bonds. According to the above illustrations, B2H4 has two types of hydrogen atoms: terminal (Ht-B) and bridging (B-Hb-B) ones, which differ in nature and characteristics. The bridging hydrogens of B2H4 participate in the electron deficient 'three-center, two-electron bonds’; thus, they bear enough partial positive charge to act as hydrogen bond donor (HBD) to form Hb...X (X = N, O) hydrogen bonds with electron donating molecules [6,7,13]. On the other hand, recent studies showed that the B-B bond also could act as HBA in the interactions of borane clusters with HBD species to form H...B-B hydrogen bonds [6,13].
From a fundamental point of view, the present work aims to extend the knowledge of the intrinsic activity of Ht, Hb and B-B bond of diborane as a hydrogen bond acceptor or hydrogen bond donor towards other molecules. For this purpose, we investigated the interaction of B2H4 with H2O, CH3OH and NHn(CH3)3-n (n = 0-3) derivatives through theoretical calculations.
Calculations were performed using the Gaussian 03 system of codes [14]. The geometries of the isolated B2H4, H2O, CH3OH, and NHn(CH3)3-n molecules as well as their complexes were fully optimized at the MP2/aug-cc-pVDZ computational level. Harmonic vibrational frequency calculations confirmed the structures as minimal and enabled the evaluation of zero point energy (ZPE). The counterpoise procedure was used to correct the interaction energy for basis set superposition error [15]. The AIMAll package was used to obtain bond properties and molecular graphs [16]. The natural bond orbitals (NBO) method was implemented within the Gaussian 03 set of codes and was applied to perform NBO analysis [17].
The atoms in molecules (AIM) theory [16] is applied here to analyze the characteristics of the H...N and H...B-B interactions through studying the location of Bond Critical Points (BCP) with (3,-1) coordinates in the Hessian matrix fitted to the intermolecular contact area. In table 1, results of the QTAIM topological parameters, namely as electronic density (ρ), Laplacian (∇2ρ) and the ratios between kinetic (G) and potential (U) electron energy density [18] are obtained. The last ones are embodied into the QTAIM formalism as follows:
H = G + U (1)
(ħ2/4m) ∇2ρ = 2G + U (2)
This equation indicates which type of interaction may exist between the two nuclei, wherein, the profile of ∇2ρ is embodied into the contribution of G and U. If the potential electron energy density is outweighed by the kinetic, the positive profile of ∇2ρ indicates a depletion of charge density along the inter-nuclear connecting Bond Path (BP) [19]. Furthermore, the atomic connection is recognized as close-shell interaction, which is often designated to H-bonds or to other intermolecular weak bound contacts, such as halogen bonds [20], dihydrogen bonds [21-23], and π-staking [24]. Regarding the values gathered in table 1, first it should be highlighted that the positive values of ∇2ρ ensure that all H-bonds are closed-shell interactions due to the low charge density concentration. The values of -G/U higher than 1, indicate that besides the non-covalent character, the N...H and H...B-B have no tend to be covalent [25].
Table 1: Topological parameters for the fully optimized complexes at MP2/aug-cc-pVDZ. View Table 1
Interaction of B2H4 with H2O and CH3OH molecules gave the B2H4-H2O and B2H4-CH3OH complexes which have hydrogen bond interactions between B-B bond as HBA and OH functions of H2O and CH3OH as HBD. Results are demonstrating that later complex has greater stability than the former one.
The association of B2H4 and NHn(CH3)3-n (n = 0-3) derivatives led to the formation of the 1:1 hydrogen bond complexes which has been denoted as B2H4-NH3, B2H4-NH2Me, B2H4-NHMe2 and B2H4-NMe3, figure 1. In these complexes hydrogen bond interactions has been found between a bridging proton of the B2H4 as a proton donor and nitrogen atom of amine as a proton acceptor (Hb...N). According to the data given in table 2, stabilities of B2H4-NHn(CH3)3-n complexes increased with enhancing basicity of amines in the following order: B2H4-NMe3 > B2H4-NHMe2 > B2H4-NH2Me > B2H4-NH3.
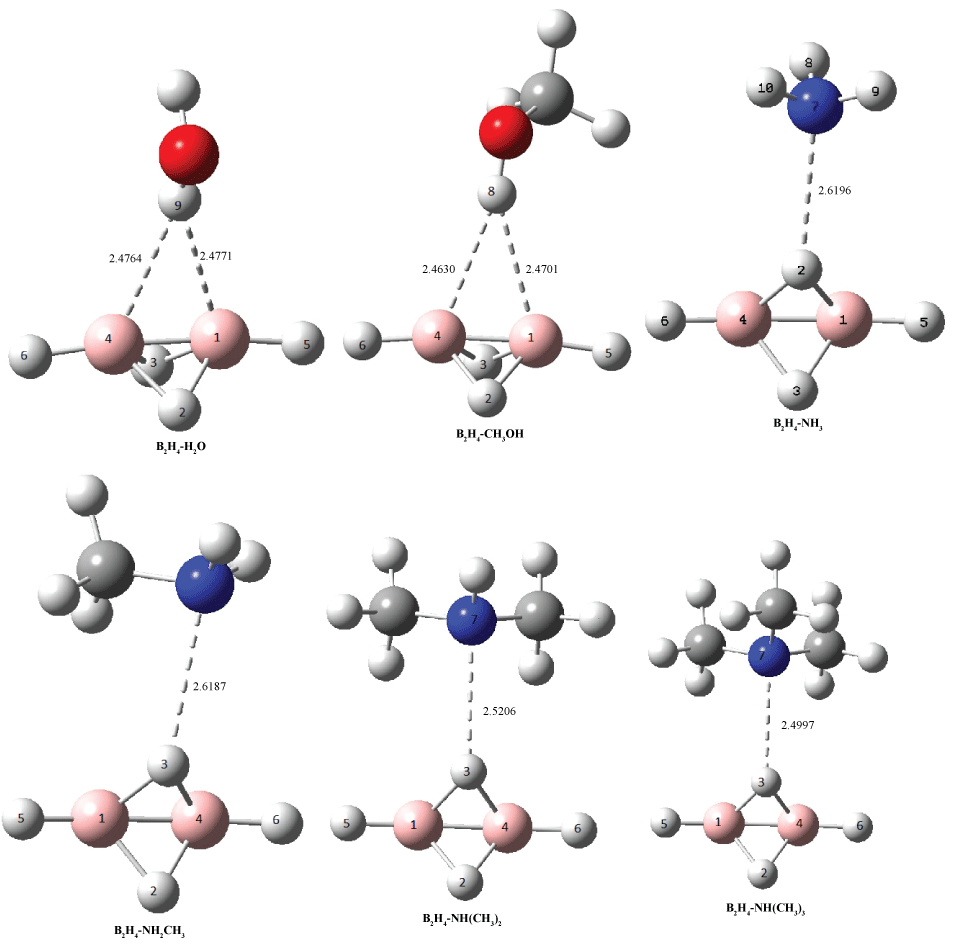 Figure 1: Schematic representation of the optimized complexes at MP2/aug-cc-pVDZ computational level. Distances are in Å. View Figure 1
Figure 1: Schematic representation of the optimized complexes at MP2/aug-cc-pVDZ computational level. Distances are in Å. View Figure 1
Table 2: The SEuncorr, BSSE, ∆ZPE, and SEcorr (corrected with BSSE and ∆ZPE) in kcal. mol-1 calculated at MP2/aug-cc-pVDZ. View Table 2
The results due to the intermolecular bond lengths are given in the table 3 and figure 1. In the B2H4-H2O and B2H4-CH3OH complexes, the B1-B4 bond has elongation (0.0015); but, other bonds of B2H4 are shortened (from -0.0009 to -0.0053) upon complex formation. Moreover, a 0.0061 lenthening was observed for O-H bond in these complexes.
Table 3: bonds length of free B2H4 and their variation during intermolecular interactions at MP2/aug-cc-pVDZ. Distances are in Å. View Table 3
On the other hand, the N...Hb distances in the B2H4-NHn(CH3)3-n complexes are in the range of 2.6196 to 2.4997 Å. These distances could be considered as weak bonding interactions between the two components. Comparison of the Hb...N distances showed that the obtaine trend was in agreement with the stability of these complexes.
In B2H4-NH3-HB, the NH3 molecule interacts with a bridging hydrogen atom of the B2H4 molecule. Data given in table 2 showed that bridging B-H-B bonds as well as B1-B4 bond were contracted (-0.0046, -0.0055, -0.0023,-0.0028 and -0.0024 for B1-H2, B4-H2, B1-H3, B4-H3 and B1-B4 bonds, respectively); while, terminal B1-H5 and B4H6 bonds designated a small elongation after complexation.
In the B2H4-NH2CH3-HB, an interaction occurs between the NH2CH3 molecule and the bridging H3 atom of B2H4. In this complex, B1-H2, B1-H3, B4-H3 and B1-B4 bonds showed contraction (-0.0077, -0.0069, -0.0023, and -0.0015, respectively); while, terminal bond B1-H5 and the bridging bond B4-H2 were elongated after complex formation.
In B2H4-NH(CH3)2-HB and in B2H4-N(CH3)3-HB, interactions occured between the bridged H3 atom of B2H4 and the amine molecules. In these complexes, B1-H2, B1-H3, B4-H2, B4-H3 and B1-B4 bonds were contracted (-0.0020 to -0.0044); while, terminal bonds of B1-H5 and B4-H6 showed small elongation after complexation.
In B2H4-H2O-HB and in B2H4-CH3OH-HB complexes, the B1-B4 bond was elongated (0.0015); but, other bonds of B2H4 were shortenned (-0.0009 to -0.0053) upon complex formation. Also, a 0.0061 Å bond lengthening was observed for O-H bond in these complexes.
The selected vibrational stretching frequencies (cm-1) with the corresponding intensities (km.mol 1) for the studied complexes are listed in table 4. In the B2H4-NHn(CH3)3-n complexes, the B1-B4 vibrational absorption band is less affected by complex formation, thus their observed shifts are negligible. But, in B2H4-H2O-HM and B2H4-CH3OH-HM complexes this bond shows -8 cm-1 red shift which is in agreement with its lengthening due to complex formation. In agreement with lengthening of B1-H5 and B4-H6 bonds, their unsymmetric stretching frequencies, which appeared at 2811 cm-1 in free B2H4, are red shifted by 6 and 9 cm-1 in B2H4-NHn(CH3)3-n complexes. In contrast, unsymmetric stretching frequencies of B1-H5 and B4-H6 showed 5 cm-1 blue shift in B2H4-H2O and B2H4-CH3OH complexes; which is in line with shortening of the related bonds. The sym-B1-H2-B4 band showed 7, 5, 15 and 15 cm-1 blue shifts in B2H4-NH3, B2H4-NH2CH3, B2H4-H2O and B2H4-CH3OH complexes, respectively; while, -6 and -9 cm-1 red shifts were observed in B2H4-NH(CH3)2 and B2H4-N(CH3)3 additives. On the other hand, unsym-B1-H2-B4 band showed 5 to 15 cm-1 blue shifts in these complexes. Moreover, the O-H band in B2H4-H2O and in B2H4-CH3OH complexes showed -40 and -127 cm-1 red shifts with respect to free H2O and CH3OH molecules, respectively.
Table 4: Unscaled vibrational frequencies (cm-1) with corresponding intensities (values given in parenthesis, km mol-1) for B2H4 and its complexes at MP2/aug-cc-pVDZ. View Table 4
Natural bond orbital (NBO) analysis was performed for the minima found on the studied B2H4 complexes. These complex formations are associated with an orbital interaction between the bonding pairs in the electron donor and the antibonding orbital in the electron acceptor. The quantity of charge transfer from donor to the acceptor (Q) due to the interaction of donor and acceptor orbitals were obtained as 0.0039, 0.0072, 0.0082, 0.0085, 0.0060 and 0.0085 for B2H4-NH3, B2H4-NH2CH3, B2H4-NH(CH3)2, B2H4-N(CH3)3, B2H4-H2O and B2H4-CH3OH complexes, respectively. The charge transfers presented above indicated that electron fraction is transferred from HBA to HBD molecule, thus charge transfer is not concentrated on the interacting atoms; but, is mostly dispersed among the molecules. Therefore, interpretation of the bond variations and frequency shifts in the B2H4 could not be carried out simply.
A useful quantity which might be derived from the results of natural bond orbital analysis is NBO binding energy (E2). The second-order perturbation energy can be taken as an index to judge the strength of the intermolecular bonds. Table 5 lists the quantity of charge transfers from donor to the acceptor qCT and the second-order perturbation energy due to the interaction of donor and acceptor orbitals. E(2) allows quantitative evaluation of the charge transfer involving in the formation of B2H4 complexes. According to the results, the E(2) value for B2H4-CH3OH was greater than for B2H4-H2O, which confirmed the order obtained for the interaction energies of these complexes. But for amine complexes, some contraversies were seen between the order obtained for their E(2) and the interaction energies.
Table 5: The NBO analysis of studied complexes at MP2/aug-cc-pVDZ. View Table 5
B2H4 has two types of terminal (Ht-B) and bridging (B-Hb-B) hydrogen atoms which differ in nature and characteristics. The bridging hydrogens of B2H4 participate in the electron deficient 'three-center, two-electron bonds’; thus, they bear enough partial positive charge to act as hydrogen bond donor (HBD) to form Hb...X (X = N, O) hydrogen bonds with electron donating molecules. The present work extended the knowledge of the intrinsic activity of Ht, Hb and B-B bond of diborane as a hydrogen bond acceptor or hydrogen bond donor towards other molecules. For this propose, the interaction of B2H4 with H2O, CH3OH and NHn(CH3)3-n (n = 0-3) derivatives thorough theoretical calculations are studied in detail.