Merkel cell carcinoma (MCC) is an aggressive form of skin cancer typically associated with a poor prognostic outcome.
A case of non-resectable, advanced Merkel cell carcinoma of the right pelvis and right inguinal region in a 67-year-old man was treated with the anti PD-L1 monoclonal antibody avelumab and Radiation therapy, with maintenance of a durable response 24 months later. The rare presentation of this aggressive form of skin cancer and its clinicopathologic features are presented.
Up-to-date information on the clinical management of MCC, including the utilization of human anti-PD-L1 monoclonal antibody, and ongoing clinical trials in both localized and metastatic settings of MCC are discussed.
Merkel cell, Avelumab, Anti-PD-L1, Cisplatin, Etoposide
Merkel cell carcinoma (MCC) is an aggressive form of skin cancer mostly seen in fair-skinned individuals between the ages of 60-80 years, and is associated with a poor prognostic outcome. Approximately 1500 new cases of MCC are diagnosed in the United States every year with an annual incidence rate of 0.7 per 100,000 persons [1]. Studies have shown high recurrence rates for local, regional and metastatic diseases [2-4]. Historically, Merkel cell carcinoma, although chemo-sensitive, remains a difficult disease to manage owing to the limited durability of available treatment modalities [5]. In fact, in patients with localized disease, recurrence rates reach 30% even after local therapy with surgery and adjuvant radiation and/or chemotherapy [4]. Until recently, no therapy has been shown to improve survival for the significant number patients with recurrent disease who are metastatic. The two-year survival rates for patients with advanced, metastatic MCC is estimated to be around 26%, making the quest for new therapeutic options extremely crucial for this patient population [6].
Although the exact pathogenesis of this very aggressive skin cancer remains elusive, certain factors have been implicated. Immunosuppression has been shown to increase the risk of Merkel cell carcinoma; for example, patients with chronic lymphocytic leukemia have a 30-fold increased risk of developing this disease, HIV patients have a 14-fold increased risk, and solid organ transplant recipients have about a 10-fold increased risk compared to general population [7]. MCC can present in various manners including as a painless, firm mass, which typically appears as a flesh-colored red or blue tumor with rapid enlargement. Approximately 50% of disease originates on sun-exposed areas of the body including head and neck and extremities, while 12% of all cases have no obvious anatomical site of origin [8]. Merkel cancer cells tend to invade locally, infiltrating the underlying subcutaneous fat, fascia or muscle, and metastasizing early in their natural history, most often to regional lymph nodes. Herein, we present a case of a patient with non resectable MCC who presented with pelvic pain secondary to a large firm mass with inguinal lymphadenopathy (Figure 1), and who achieved durable remission after immune checkpoint blockade treatment.
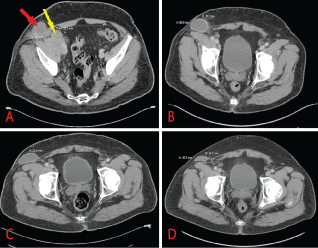 Figure 1: CT scan of abdomen/pelvis with contrast. (a) Right pelvic mass measuring 5.2 × 4.9 × 5.5 cm (yellow arrow) and inguinal lymph node measuring 4.9 cm in diameter (red arrow) at diagnosis; (b) 3 months following initiation of avelumab therapy; (c) 6 months post avelumab treatment; (d) 9 months post avelumab treatment. View Figure 1
Figure 1: CT scan of abdomen/pelvis with contrast. (a) Right pelvic mass measuring 5.2 × 4.9 × 5.5 cm (yellow arrow) and inguinal lymph node measuring 4.9 cm in diameter (red arrow) at diagnosis; (b) 3 months following initiation of avelumab therapy; (c) 6 months post avelumab treatment; (d) 9 months post avelumab treatment. View Figure 1
A 67-year-old male with past medical history significant for diabetes type 2, hypertension, hyperlipidemia, obstructive sleep apnea, metabolic syndrome with morbid obesity status post lap sleeve gastrectomy presented with right lower quadrant abdominal pain. A computed tomography (CT) scan of the abdomen/pelvis with intravenous and oral contrast revealed a solid-appearing soft tissue mass in the right lower quadrant as well as a low-attenuation, peripherally enhancing structure in the right groin, raising suspicion for malignancy with lymphadenopathy. The overall dimensions of the mass were approximately 5.2 × 4.9 × 5.5 cm. In the right groin the peripherally enhancing, cystic-appearing lesion measured 4.9 × 4.6 × 6.4 cm (Figure 1). A CT of the chest showed no evidence of primary or metastatic malignancy (not shown). MRI of the brain with and without gadolinium did not show any overt mass or pathologic intracranial enhancement (Figure 2). A positron emission tomography/computed tomography (PET/CT) scan showed a large FDG-avid soft tissue mass anterior to the right iliopsoas along the right external iliac chain with a maximum standardized uptake value (SUV) of 17.0. An adjacent right inguinal mass with central necrosis had a maximum SUV of 15.2 (Figure 3). Ultrasound-guided biopsy was performed, with final pathology determination of as mall blue cell tumor with extensive necrosis favoring Merkel cell carcinoma based on immunohistochemistry. The tumor cells stained positively for CK20, CD 56 and Synaptophysin and negatively for TTF-1 (Figure 4). Merkel cell polyomavirus (MCPyV) status was not assessed.
 Figure 2: MRI brain w/wo contrast: Sagittal T1 FLAIR, Axial T2 FLAIR, Diffusion-weighted, Axial T2, Proton density, Pre- and post-contrast FSPGR, Post-contrast spin-echo T1 using a 3 Tesla GE MR scanner. View Figure 2
Figure 2: MRI brain w/wo contrast: Sagittal T1 FLAIR, Axial T2 FLAIR, Diffusion-weighted, Axial T2, Proton density, Pre- and post-contrast FSPGR, Post-contrast spin-echo T1 using a 3 Tesla GE MR scanner. View Figure 2
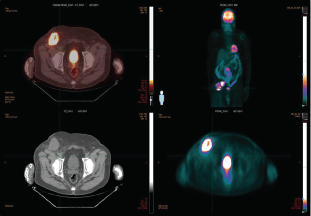 Figure 3: PET/CT showing large FDG avid soft tissue mass anterior to the right iliopsoas along the right external iliac chain with a max SUV 17.0. An adjacent right inguinal mass with central necrosis has a max SUV of 15.2 at diagnosis. View Figure 3
Figure 3: PET/CT showing large FDG avid soft tissue mass anterior to the right iliopsoas along the right external iliac chain with a max SUV 17.0. An adjacent right inguinal mass with central necrosis has a max SUV of 15.2 at diagnosis. View Figure 3
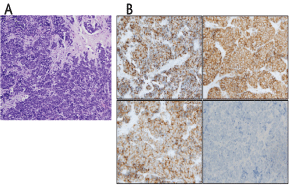 Figure 4: Histopathologic evaluation of sections of the right groin mass. (a) A proliferation of small, uniform, round to polygonal cells with scant cytoplasm and finely granular nuclei. Nuclear molding and necrosis are present (Hematoxylin & Eosin, 200x magnification); (b) Immunohistochemistry for CK20 shows the characteristic perinuclear dot like positivity (upper left image). Tumor cells were also positive for CD56 and synaptophysin (upper right image and lower left image respectively). TTF-1 staining was negative in the tumor cells (lower right image). All images, 200x magnification. View Figure 4
Figure 4: Histopathologic evaluation of sections of the right groin mass. (a) A proliferation of small, uniform, round to polygonal cells with scant cytoplasm and finely granular nuclei. Nuclear molding and necrosis are present (Hematoxylin & Eosin, 200x magnification); (b) Immunohistochemistry for CK20 shows the characteristic perinuclear dot like positivity (upper left image). Tumor cells were also positive for CD56 and synaptophysin (upper right image and lower left image respectively). TTF-1 staining was negative in the tumor cells (lower right image). All images, 200x magnification. View Figure 4
At the time of diagnosis, imaging studies showed encasement of the femoral vessels making the tumor non-resectable. The patient underwent treatment with immunotherapy and concurrent radiotherapy (60Gy). Systemic treatment with immunotherapy consisted of the then newly approved anti-PD-L1 monoclonal antibody avelumab (Bavencio, Merck KGaA); intravenous 10 mg/kg every two weeks. Patient received premedication with an antihistamine prior to the first three infusions after which no more premedication was given. Systemic treatment was given concomitantly with radiotherapy. Radiation treatment was initiated approximately 3 weeks following the first administration of immunotherapy. Based on recommended frequency of tumor reassessment in patients treated with immunotherapy, follow up imaging was performed 12 weeks following initial treatment and repeated at 3 month intervals to assess response. Patient is still receiving avelumab at 10 mg/kg every two weeks. Plan of treatment is to continue immunotherapy until disease progression or unacceptable toxicity. The patient showed significant and progressive improvement in tumor resolution at 3, 6 and 9 months intervals (Figure 1); based on immunotherapy-response RECIST (iRECIST) criteria, this patient has immune-stable disease (iSD). The previously encased femoral vessels could be visualized 3 months following initiation of immunotherapy (Figure 1B). The patient continues to receive immunotherapy with complete resolution of symptoms. In further follow up with our clinic, he has had no new complaints or immune related adverse event at approximately 24 months since initiation of immunotherapy.
Diagnostic approaches for MCC includes immunohistologic evaluation of tissue biopsy. This is extremely crucial so as to differentiate MCC from other morphologically similar tumors such as small cell lung cancer, small cell variant of melanoma, various cutaneous leukemic/lymphoid neoplasms as well as Ewing's sarcoma. Histopathologically, cytokeratin 20 (CK20) and thyroid transcription factor I (TTF-1) evaluation must be included to help exclude small cell lung cancer. CK20 is a very sensitive marker for Merkel cell carcinoma with sensitivity range between 89%-100%, whereas TTF 1 is expressed in 83% to 100% of small cell lung cancers but is usually absent in Merkel cell carcinoma [9,10].
Sentinel lymph node biopsy is the most sensitive staging test to detect nodal diseases, although the overall impact of nodal status on survival remains unclear. The recurrence rate for MCC was reported at 56% in sentinel lymph node biopsy-positive patients and 39% in sentinel lymph node biopsy negative-patients; however, identifying patients with positive microscopic nodal disease and then performing full lymph node dissection and/or radiation therapy maximizes the care for regional disease [11,12].
Imaging modalities such as PET/CT, CT scan or MRI are useful in identifying distant metastases. Recently PET/CT scan has gained importance in the diagnosis of Merkel cell carcinoma and may be preferred in certain circumstances, as it can identify bone metastases that are not detected by CT scan. For instance, in a recent review involving over 100 patients, PET/CT results changed the stage and thus the primary treatment of 22% of patients and altered the radiation technique/doses in another 15% of patients [11].
Once diagnosis has been established, surgery with negative margins remains the primary treatment strategy in localized Merkel cell carcinoma. Given the high recurrence rate of up to 30%, wide local excision with 1-2 cm margins to the investing fascia layer remains the standard [12]. There have been mixed reports regarding the benefits of radiation therapy with some studies showing evidence of reduced local regional recurrence following postoperative radiation therapy. In one meta-analysis that compared surgery alone versus surgery plus adjuvant radiation, the use of local adjuvant radiation after complete excision decreased the risk of local and regional recurrence [13].
While surgery and/or radiation therapy are utilized in resectable MCC, there is no consensus on management of metastatic MCC. This could be due to the rarity of MCC in general. Studies show that approximately 10% of patients present with stage IV MCC and among those presenting with local disease, 33% develop distant metastatic disease [14]. Metastatic sites are diverse and include but are not limited to liver, lung, lymph nodes, subcutaneous tissue, pancreas, heart, and parotid gland. Approximately 10% will develop brain metastasis [15]. Historically, survival for patients presenting with advanced, metastatic MCC has been dismal, with a median survival of approximately 1 year and almost 100% chance of death by 5 years [14,16].
Until recent breakthroughs in the area of immunotherapy, cytotoxic chemotherapy with or without surgery and/or radiation therapy remained the first line therapy for stage IV disease. Although these chemotherapeutic agents offered a high objective response rate (ORR), the durability of disease control was poor (median progression-free survival [PFS] of approximately 3 months and median duration of response ≤ 8 months) [17,18]. In patients that progressed after first line therapy, subsequent lines of treatment were ineffective, with an ORR of 10% and median PFS of only 1.9 months [17-19].
These earlier studies investigated chemotherapy regimens similar to those used for small cell lung cancer, because of its neuroendocrine differentiation and histopathologic features [20,21]. George, et al. reported a progression-free survival benefit following a single treatment with carboplatin [20]. Following that publication, several case reports and series presented therapeutic outcomes following single chemotherapeutic treatments or combined treatment with radiation therapy [22-27]. Chemotherapeutic agents such as carboplatin, cisplatin, 5-FU, cyclophosphamide, doxorubicin (or epirubicin), vincristine plus or minus prednisone, and etoposide were utilized among this patients subgroup. Unfortunately, these agents have not been shown to improve overall survival in patients with metastatic MCC.
Few studies with newer targeted systemic therapeutic strategies are available due to the rarity of the disease. In one of the earlier studies on targeted therapy, Brunner, et al. reported increased expression of therapeutically useful targets such as c-kit, Bmi-1, Mcl-1, VEGF-A and VEGF-C, VEGF-R2, PDGF-α and PDGF-β [28]. These promising results further validate clinical studies utilizing multitargeted tyrosine kinase inhibitors and antisense oligonucleotides in MCC [28]. Other studies targeting merkel cell polyomavirus (MCPyV) also showed promising results in patients with Merkel cell carcinoma [29,30].
MCC is a highly immunogenic virus-associated malignancy, with MCPyV detectable in 43%-100% of patients [31]. In addition to clonal integration of MCPyV, this aggressive skin cancer has also been associated with immunosuppressive state (especially post-transplant), lymphoproliferative disorders and old age [32]. Although the role of MCPyV in disease pathogenesis remains unclear, patients with MCPyV-positive tumors were shown to have improved overall survival compared to those with MCPyV-negative tumors [33]. The mechanism of malignant transformation via MCPyV in patients with MCC is activation of the cascades involved in the generation of antigen-specific T cells and antibodies [17,34]. Programmed death 1 (PD-1), the major receptor for programmed death-ligand 1 (PD-L1), is expressed by activated T lymphocytes, B cells and myeloid cells, and the receptor/ligand complex plays a major role in T cell inhibition versus reactivation of the immune system against malignancy [35,36]. In a cohort of 49 patients, Lipson, et al. reported increased expression of PD-L1 in the MCC microenvironment of patients with MCPyV-positive disease but not MCPyV-negative. They also showed that tumor cell expression of PD-L1 was an independent positive prognostic factor [37].
These findings provided the rationale for a novel immunotherapeutic approach in patients with metastatic MCC, utilizing PD-1/PD-L1 immune checkpoint blocking. Avelumab, an anti-PD-L1 monoclonal antibody, was approved in 2017 by US Food and Drug Administration as a breakthrough therapy for metastatic Merkel Cell Carcinoma, and was the first FDA-approved product to treat this type of cancer [38]. The pivotal JAVELIN Merkel 200 study (NCT02155647) was a single-arm, multi-center prospective clinical trial involving 88 patients with metastatic Merkel cell carcinoma who had previously been treated with chemotherapy that included a platinum-based agent (cisplatin or carboplatin) ± etoposide; cyclophosphamide + doxorubicin + vincristine; topotecan; gemcitabine; irinotecan; paclitaxel; nab-paclitaxel; or docetaxel. Atmedian follow-up of 16.4 months patients had an overall response rate of 33% (95% confidence interval (CI) [23.3, 43.8]). Most interestingly, all of the patients who responded to the immunotherapy initially continued to respond with most responses lasting more than 1 year [39]. Subgroup analyses showed trends for higher objective response rate (ORR) in patients who received fewer prior lines of anticancer treatment (1 vs. ≥ 2 prior lines, 40.4% vs. 22.2%), who had lower disease burden (sum of target lesion diameters ≤ median vs. > median, 41.0% vs. 26.3%), and in tumors that had positive PD-L1 expression [39].
Furthermore, the recently updated efficacy data in patients with metastatic MCC treated with avelumab with more than 1 year follow-up continues to show durable responses and promising survival outcome even in patients who had disease progression after prior chemotherapy and those with PD-L1 positive tumors irrespective of MCPyV status [39]. Treatment-related (TR) adverse events (AEs) occurred in 59 patients (67.0%); those occurring in ≥ 10% of patients were fatigue (21.6%) and infusion-related reaction (13.6%). Grade 3 TRAEs were reported in 3 patients (3.4%); there were no grade 4 TRAEs or treatment-related deaths [39].
The durable response seen in the JAVELIN Merkel 200 study was also exemplified by our patient with non-resectable Merkel cell carcinoma who has shown durable response to avelumab approximately 24 months following initial treatment. He continues to be seen regularly in our clinic and has so far tolerated treatment well with reports only of mild fatigue.
Currently there are clinical trials investigating the efficacy of other checkpoint blockade treatments, combination treatments with a checkpoint inhibitor backbone, and novel agents (Table 1). There is an ongoing trial with ipilimumab (anti-CTLA-4) in the adjuvant setting after resection of local MCC disease (NCT02196961). There is also a phase II clinical trial underway studying the combination nivolumab and ipilimumab versus nivolumab or ipilimumab and stereotactic body radiation therapy for metastatic MCC (NCT03071406) (Table 1). One novel adoptive cell therapy being investigated is the use of Talimogene laherparepvec, a genetically modified herpes simplex virus-type 1 attenuated by deletion of the herpes neuro-virulence viral genes, and enhanced for immunogenicity. This highly immunogenic virus has been used to treat patients with advanced MCC with complete response reported within 2 months of first injection [17]. There is currently a phase II clinical trial (NCT02978625) underway studying Talimogene laherparepvec in combination with nivolumab in the metastatic setting.
Table 1: Ongoing Clinical trials in MCC. View Table 1
The use of Merkel cell polyomavirus-specific T cells as a treatment for MCC either alone or in combination with interleukin-2 and human leukocyte antigen (HLA)-I upregulating agents (single-dose radiation and interferon-beta injections) have been reported [40]. A phase I/II trial investigated Merkel cell polyomavirus-specific T cells in combination HLA upregulation (radiation or interferon) with (i.e., triple therapy) and without (i.e., double therapy) avelumab [41]. In this trial, four patients received triple therapy and three of the four patients had sustained complete response (NCT02584829). One out of the four patients who received double therapy had complete response before progression at 14 months, but the other three patients had progression of disease. Apart from Avelumab, other anti-PD-L1antibodiesare being investigated. An ongoing phase II clinical trial investigating patients with Merkel cell carcinoma who were treated with the anti-PDL 1 monoclonal antibody pembrolizumab showed 56% complete or partial response among treated patients (NCT02267603) [42]. Data acquisition and analysis from the trial is currently ongoing.
During the recently concluded 2018 american association of clinical oncology (ASCO) conference, data was presented from a multicenter, phase I/II Checkmate-358 trial (NCT02488759), which investigated the safety and tolerability of neoadjuvant nivolumab in patients with advanced, virus-associated cancers [43]. In their study, 15 of 21 evaluable patients (71%) were MCPyV positive and 6 of 20 evaluable patients (30%) were PD-L1 positive (≥ 1% cutoff). Both groups of patients were treated with 240 mg of nivolumab intravenously on days 1 and 15, with surgery planned for day 29. The median follow-up was 67.1 weeks after the initial dose of nivolumab. Twelve patients (41%) had TRAEs, and two patients (7%) had grade 3/4 TRAEs. The most commonly reported TRAEs were fatigue and nausea. The investigators further reported that among the 25 evaluable patients, the median change in radiographic tumor reduction was -19.2% (range, -100 to 73) and responses were observed regardless of MCPyV or PD-L1 positivity. Finally, the authors reported a progression free survival (PFS) rate of 72.6% (95% CI [48.6, 86.8]) and overall survival (OS) of 75.0% (95% CI [31.5, 93.1]); the median PFS and median OS have not been reached [39].
Generally, MCC is a chemo-sensitive disease; however, the durability of response has been found to be transient. Data emerging from immune checkpoint blockade studies are encouraging. Our patient who presented with non-resectable MCC responded well to immune checkpoint blockade and has maintained durability of response since initiation of immunotherapy approximately 24 months ago, with complete resolution of symptoms. Several clinical trials of immune checkpoint inhibitors are ongoing and potentially will provide better treatment options for patients with both localized and metastatic MCC in the near future, with the ultimate goal of bringing these effective treatments to the front line in the adjuvant setting. Given the impressive results of immune checkpoint blockade in the metastatic setting, we encourage clinicians to enroll patients with MCC into clinical trials evaluating the effectiveness of these immunotherapies in the adjuvant setting. Furthermore, MCPyV status, which has been shown to provide both prognostic and predictive value, should be obtained as a diagnostic tool in the workup of MCC patients, since the majority of MCPyV-positive patients will express PD-L1, linked to improved overall survival.