The introduction of corticosteroids in the therapeutic armamentarium has been a great contribution to human health. Budesonide, a semi-synthetic glucocorticoid derivative gained its popularity due to its anti-inflammatory, immunosuppressive and even anti-carcinogenic properties. In this study we examine the effect of budesonide on the immune dialogue between peripheral blood mononuclear cells and colon carcinoma cells from two human lines.
Unstimulated peripheral blood mononuclear cells or cells stimulated with either lipopolysaccharide or phorbol myristate acetate/ionomycin were incubated without or with budesonide at 10-8 M, 10-7 M and 10-6 M and the production of TNFα, IL-1β, IL-6, IFNγ, IL-2, IL-1ra and IL-10 was examined applying ELISA method. In another set of experiments peripheral blood mononuclear cells were co-cultured with HT-29 or RKO colon carcinoma cells in the absence or presence of budesonide and the secretion of the abovementioned cytokines was evaluated.
Budesonide caused reduced proliferation of peripheral blood mononuclear cells, HT-29 or RKO at 10-8 M as tested by XTT test, but had no effect at higher concentrations. The secretion of TNFα, IL-1β and IL-6 by non-stimulated peripheral blood mononuclear cells or cells stimulated with either lipopolysaccharide or cells from both colon cancer lines was inhibited upon incubation with budesonide at concentrations between 10-8 M and 10-6 M. The production of IL-2 by phorbol myristate acetate/ionomycin treated peripheral blood mononuclear cells or cells stimulated with HT-29 colon cancer cells was suppressed by budesonide, but IL-2 secretion by RKO stimulated peripheral blood mononuclear cells was not affected. Budesonide did not influence the secretion of IFNγ by non-stimulated peripheral blood mononuclear cells or by cells stimulated with PMA/ionomycin. However, HT-29 and RKO-induced production of IFNγ was inhibited by the three budesonide concentrations. Budesonide caused reduced secretion of IL-10 by non-stimulated peripheral blood mononuclear cells, or by cells stimulated with either lipopolysaccharide or RKO cancer cells, whereas that induced by HT-29 cells was not affected. IL-1ra synthesis by non-stimulated peripheral blood mononuclear cells or cell stimulated with HT-29 colon cancer cells was inhibited upon 24 hrs incubation with budesonide, while that produced by lipopolysaccharide or RKO stimulated PBMC was not affected.
It appears that the capacity of budesonide to modify the immune dialogue between mononuclear and certain types of cancer cells may be one of the ways by which this glucocorticosteroid may affect tumorigenesis.
Budesonide, Cytokines, Mononuclears, Colon cancer cells
Glucocorticosteroids as therapeutic agents triggered a dramatic revolution in the treatment of a long list of medical conditions due to their prodigious pleiotropic effects [1,2], and have been designated as some of the most important drugs used in the everyday clinical practice [3]. The mechanisms of activities, the mode of alleviation of suffering and recovery of diseases achieved by their administration have been meticulously reviewed [4,5]. The beneficial effect of glucocorticosteroids on chronic inflammation and autoimmune diseases indicates existence of a close relationship with the immune system. There is compelling evidence that at pharmacological doses glucocorticosteroids may alter both cellular and humoral immunity concurrently with capacities to modulate additional functions of the white blood cells, qualities that promoted them as the mainstay of immunosuppressive agents [6,7]. A significant part of the glucocorticosteroids' immunomodulatory activity is own to their ability to interfere with the complex functions of the macrophages, mainly by their capacity to produce inflammatory cytokines [8]. This attribute is applied for treatment of chronic inflammatory diseases such as asthma, rheumatoid arthritis, inflammatory bowel and autoimmune diseases [9,10]. It is of interest that although glucocorticosteroids are widely accepted as anti-inflammatory agents they exert also pro-inflammatory activities [11] that may explain the predisposition to infections in patients treated for longer periods [12]. Pretreatment of murine macrophages with glucocorticosteroids induced increased production of TNFα and IL-6, recognized as pro-inflammatory cytokines [10]. Therefore, a studious tendency exists for development of novel semi-synthetic corticosteroids with lesser undesirable properties. One of these products with pronounced anti-inflammatory properties is budesonide, widely used for treatment of asthma. Studies have shown that budesonide may express anti-inflammatory effect also on intestinal epithelial cells explaining its beneficial activity in inflammatory bowel disease and in microscopic colitis [13,14]. Based on the well-established fact that there is a close relationship between chronic inflammation and tumor development [15], investigations have been initiated as for the chemopreventive effect of corticosteroids on tumorigenesis [16]. However, due to the side effects following their prolonged administration, the attention has been diverged to the anti-cancer effect of the semi-synthetic corticosteroids such as budesonide [17]. According to Wattenberg, et al. [18,19] budesonide exerted a marked concentration-dependent suppressive activity on pulmonary tumor formation in mice. In previous studies we have shown the existence of a cross-talk between peripheral blood mononuclear cells (PBMC) and colon cancer cells [20]. In the present work we brought up the question if budesonide may alter the immune cross-talk between human PBMC and cells from two human colon carcinoma lines, i.e. HT-29 and RKO.
Buffy coats obtained from donors' blood were purchased from Magen David Adom, Blood Services Center in Israel after signing an informed consent containing a written agreement that blood components not suitable for therapeutic needs can be used for medical research. PBMC were separated by Lymphoprep-1077 (Axis-Shield PoC AS, Oslo, Norway) gradient centrifugation. The cells were washed twice in phosphate buffered saline (PBS) and suspended in RPMI-1640 medium containing 1% penicillin, streptomycin and nystatin, 10% fetal bovine serum (FBS, Biological Industries, Beit Haemek, Israel) and was designated as complete medium (CM).
HT-29 and RKO human colon cancer cell lines were obtained from American Type Cultural Collection, Rockville, MD. The cells were maintained in CM containing Mc-Coy's 5A medium and Dulbecco modified eagle medium (DMEM) respectively, supplemented with 10% FBS, 2 mM L-glutamine and antibiotics (penicillin, streptomycin and nystatin-Biological Industries Co, Beit-Haemek, Israel). The cells were grown in T-75 culture flasks at 37 ℃ in a humidified atmosphere containing 5% CO2. Since the cells from both lines adhere to the flasks they were removed using trypsin/EDTA solution (Biological Industries Co, Beit-Haemek, Israel).
Budesonide (Sterinebs Teva) was purchased from Teva Pharmaceutical Industries, Ltd, Israel at a concentration of 0.5 mg/ml. Further dilutions were prepared in CM. The drug was added at the onset of cultures at final concentrations of 10-6 M, 10-7 M and 10-8 M.
The effect of budesonide on PBMC and colon cancer cells proliferation was determined using XTT proliferation assay kit (Biological Industries, Beit Haemek, Israel). Briefly: 0.1 ml aliquots of PBMC or colon cancer cells (105/ml of CM) obtained after trypsinization and suspended in appropriate CM, were added to each one of 96 well plates and incubated for 24 hrs in the absence or presence of budesonide at concentrations as indicated. At the end of the incubation period the cells were stained according to the manufacturer's instructions. The plates were incubated for 2-4 hrs at 37 ℃ in a humidified incubator containing 5% CO2 and the absorbance was measured at 450 nm using ELISA reader.
2 × 106 MC suspended in 1 ml of CM were incubated in 24 well plated (Nunc, Thermo Fisher Scientific, Paisley PA4 9RF, UK) without (control) or with budesonide added at the onset of the cultures at final concentrations as indicated. Cultures were incubated with 50 ng/ml of lipopolysaccharide (LPS, E. coli, Sigma) to determine the effect of budesonide on TNFα, IL-1β, IL-6, IL-1ra and IL-10 and with PMA 1 µg/ml and ionomycin 0.5 µg/ml (Sigma, Israel) for IL-2 and IFNγ secretion. In another set of experiments, HT-29 or RKO cells were collected from the flasks by trypsinization and suspended in the appropriated CM at 4 × 105/ml. The cells were seeded in 24 well plates by addition of 0.5 ml of cell suspension to each well. The plates were incubated for 60 min at 37 ℃ in a humidified atmosphere containing 5% CO2 before adding 0.5 ml aliquots of PBMC suspended in CM (4 × 106/ml) to each well. Cells were incubated without or with budesonide added at the onset of cultures at concentrations as indicated. All cultures were maintained for 24 hrs at 37 ℃ in a humidified atmosphere containing 5% CO2. At the end of the incubation period, the culture media were collected, the cells were removed by centrifugation at 200 g for 10 min and the supernatants were kept at -70 ℃ until assayed for cytokine content.
The concentration of cytokines in the supernatants was tested using ELISA kits specific for human cytokines (BioLegend Inc., San Diego, CA) as detailed in the guide-line provided by the manufacturer. The catalogue numbers for capture antibodies for TNFα-BLG 502802, IL-1β- BLG 508202, IL-6- BLG 501101, IL-1ra- BLG 509901, IL-10- BLG 501402, IL-2- BLG 500302 and IFNγ- BLG 502401 and for the detection biotin antibodies for TNFα-BLG 502904, IL-1β- BLG 508302, IL-6- BLG 501201, IL-1ra- BLG 509502, IL-10- BLG 501501, IL-2- 13-7027-81 and IFNγ- BLG 502504. The detection level of all cytokines was 30 pg/ml.
A linear mixed model with repeated measures and assumption of compound symmetry (CS) was used to assess the effect of different concentrations of budesonide on cytokine secretion by non-stimulated or stimulated PBMC. SAS vs. 9.4 was applied for this analysis. Paired t-test was used to compare between the level of cytokines produced following incubation with various concentrations of budesonide and that found in control cultures. Probability values of p < 0.05 were considered as significant. The results are expressed as mean ± SEM.
24 hrs of incubation of PBMC or HT-29 and RKO colon cancer cells with budesonide at concentrations between 10-8 M and 10-6 M caused inhibition of cell proliferation as tested by XTT assay (p = 0.015, p = 0.006 and p = 0.0005, respectively (Table 1). Budesonide added to PBMC, HT-29 or RKO at 10-8 M caused reduced proliferation by 10% (p = 0.006), 12% (p = 0.005) and 18% (p < 0.05) respectively. At higher concentrations of budesonide, the proliferation rate of the PBMC and the HT-29 colon cancer cells was not affected whereas that of RKO cells was enhanced by 18% (p = 0.02) at 10-6 M budesonide.
Table 1: Effect of budesonide on cell proliferation. View Table 1
Supernatants obtained from 24 hrs cultures of either HT-29 or RKO cells incubated without or with budesonide at concentrations between 10-8 M and 10-6 M did not contain detectable amounts of any of the cytokines tested.
The secretion of TNFα, IL-1β and IL-6 by non-stimulated PBMC or cells stimulate with either LPS or cells from both colon cancer lines was inhibited upon incubation with budesonide at the above mentioned concentrations (p < 0.001 for TNFα and IL-1β; p < 0.01 for IL-6. Figure 1, Figure 2 and Figure 3 respectively). Inhibition of TNFα, IL-1β and IL-6 by non-stimulated PBMC was maximally achieved at budesonide concentration of 10-8 M (32%, 43% and 50%, respectively). TNFα, IL-1β and IL-6 production by LPS-stimulated PBMC or cells stimulated with either HT-29 or RKO cells was maximally reduced at budesonide concentration of 10-7 M (45%, 32% and 36%, respectively for TNFα; 38%, 37% and 30%, respectively for IL-1β and 17%, 16% and 25%, respectively for IL-6).
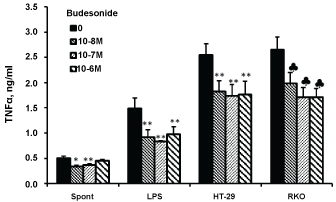 Figure 1: Effect of budesonide on TNFα production.
Figure 1: Effect of budesonide on TNFα production.
Non-stimulated PBMC (spontaneous) or cells stimulated with either LPS or with HT-29 or RKO colon cancer cells, were incubated for 24 hrs without (0) or with budesonide at concentrations as indicated. The level of TNFα in the supernatants was tested by ELISA. Each bar expresses the mean results of 6 experiments. Bars represent SEM. Symbols represent statistically significant difference from cells incubated without budesonide (*p < 0.05; **p < 0.01; ♣p < 0.001). View Figure 1
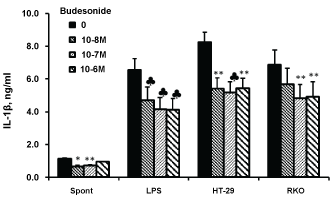 Figure 2: Effect of budesonide on IL-1β production.
Figure 2: Effect of budesonide on IL-1β production.
Non-stimulated PBMC (spontaneous) or cells stimulated with either LPS or with HT-29 or RKO colon cancer cells, were incubated for 24 hrs without (0) or with budesonide at concentrations as indicated. The level of IL-1β in the supernatants was tested by ELISA. Each bar expresses the mean results of 6 experiments. Bars represent SEM. Symbols represent statistically significant difference from cells incubated without budesonide (*p < 0.05; **p < 0.01; ♣p < 0.001). View Figure 2
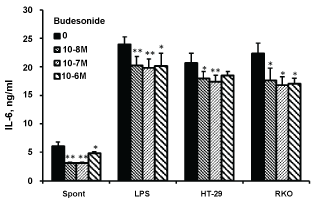 Figure 3: Effect of budesonide on IL-6 production.
Figure 3: Effect of budesonide on IL-6 production.
Non-stimulated PBMC (spontaneous) or cells stimulated with either LPS or with HT-29 or RKO colon cancer cells, were incubated for 24 hrs without (0) or with budesonide at concentrations as indicated. The level of IL-6 in the supernatants was tested by ELISA. Each bar expresses the mean results of 6 experiments. Bars represent SEM. Symbols represent statistically significant difference from cells incubated without budesonide (*p < 0.05; **p < 0.01). View Figure 3
No detectable levels of IL-2 could be detected in supernatants of non-stimulated PBMC or cells incubated for 24 hrs with budesonide at the above mentioned concentrations (Figure 4). IL-2 production by PMA/ionomycin treated PBMC or cells stimulated with HT-29 colon cancer cells was suppressed by 51% and 32%, respectively (p < 0.001), upon 24 hrs of incubation with budesonide, but IL-2 secretion by RKO stimulated cells was not affected. Budesonide did not influence the secretion of IFNγ by non-stimulated PBMC or by cells stimulated with PMA/ionomycin. However, HT-29 and RKO-induced production of IFNγ was inhibited by the three budesonide concentrations and was maximally reduced by 30% and 60%, respectively (p < 0.001).
 Figure 4: Effect of budesonide on IFNγ and IL-2 production.
Figure 4: Effect of budesonide on IFNγ and IL-2 production.
PBMC stimulated with either PMA/ionomycin or with HT-29 or RKO colon cancer cells, were incubated for 24 hrs without (0) or with budesonide at concentrations as indicated. The level of IFNγ and IL-2 in the supernatants was tested by ELISA. Each bar expresses the mean results of 6 experiments. Bars represent SEM. Symbols represent statistically significant difference from cells incubated without budesonide (*p < 0.05; ♣p < 0.001). View Figure 4
Reduced secretion of IL-10 by non-stimulated PBMC (p < 0.001), as well as by cells stimulated with either LPS (p < 0.01) or RKO cancer cells (p < 0.05) was found when cultures were incubated with budesonide at concentrations between 10-8 and 10-6 M, whereas that induced by HT-29 cells was not affected (Figure 5). IL-10 production by non-stimulated or LPS stimulated PBMC was lowered by 33% and 28% respectively at 10-7 M budesonide and that induced by RKO cells was reduced by 15%. IL-1ra synthesis by LPS or RKO stimulated PBMC was not affected by 24 hrs. of incubation with various budesonide concentrations. However, IL-1ra production by non-stimulated PBMC or cell stimulated with HT-29 colon cancer cells was inhibited (p < 0.001) upon 24 hrs incubation with budesonide and was lower by 56% and 22% at drug concentrations of 10-8 and 10-7 M, respectively.
 Figure 5: Effect of budesonide on IL-10 and IL-1ra production.
Figure 5: Effect of budesonide on IL-10 and IL-1ra production.
Non-stimulated PBMC (spontaneous) or cells stimulated with either LPS or with HT-29 or RKO colon cancer cells, were incubated for 24 hrs without (0) or with budesonide at concentrations as indicated. The concentration of Il-10 and IL-1ra in the supernatants was tested by ELISA. Each bar expresses the mean results of 6 experiments. Bars represent SEM. Symbols represent statistically significant difference from cells incubated without budesonide (*p < 0.05; ♣p < 0.001). View Figure 5
Monocytes, transforming to macrophages into tissues are active participants in the process of inflammation [21]. Once stimulated, they modulate inflammatory events by an array of activities including cytokine production. Incubation of unstimulated PBMC with budesonide in the present study caused a decreased production of the proinflammatory cytokines TNFα, IL-1β and IL-6, although the secretion of IFNγ and IL-2 was not affected. Interestingly, this activity was observed when the cells were incubated with lower budesonide concentrations. A similar response was detected when the production of the anti-inflammatory cytokines IL-10 and IL-1ra was assessed, with exception that the secretion of the latter was inhibited by all three concentrations of budesonide. One possible explanation for this phenomenon is that once PBMC have been maximally inhibited for certain cytokine production by budesonide the cells cannot further respond to its higher concentrations. When PBMC were stimulated with LPS or PMA the secretion of all pro-inflammatory cytokines examined was inhibited at all budesonide concentrations applied throughout the experiments. As for the anti-inflammatory cytokines, the secretion of IL-10 and IL-1ra by unstimulated PBMC was lowered. However budesonide caused reduced IL-10 production by LPS stimulated cells, whereas that of IL-1ra was not affected. This observation is in accord with other reports revealing that LPS activated human monocytes show a suppressed production of IL-1 and IL-6 after incubation with budesonide [22,23]. It has been observed that budesonide reduces immune responses by inhibition of IL-12, IL-6, IL-8 and TNFα release by PBMC from asthmatic and healthy donors [24-27]. Rudiger, et al. [28] have reported a significant reduction in IL-1β, IL-6, IL-8 and TNFα secretion by LPS induced lymphocytes isolated from blood of healthy and mild asthmatic individuals following budesonide inhalation. Notably, budesonide has been able to affect cytokine production by other cell types, such as human mast cells in which IL-1β, IL-4, IL-6 and TNFα was found to be inhibited [29] and by rat alveolar macrophages that showed decreased IL-6 and TNFα secretion [30]. Previously, we have demonstrated that the immune cross-talk between PBMC and HT-29 and RKO colon cancer cells may be modified by a number of substances [31]. Correspondingly, budesonide added to co-cultures of PBMC with cells of each one of the colon cancer lines utilized in the present study caused a modulation of the immune relationship between mononuclear and malignant cells. Budesonide markedly reduced the ability for pro-inflammatory cytokine production by PBMC incubated with HT-29 cells. The production of IL-1ra was inhibited, whereas that of IL-10 was not affected. At the same laboratory settings addition of budesonide to co-cultures of PBMC with RKO cells produced a slightly different effect. The secretion of all pro-inflammatory cytokines was inhibited except for IL-2. The production of the anti-inflammatory cytokine IL-1ra was reduced but that of IL-10 was not affected. One conceivable explanation for this observation is that budesonide wields concentration and cell-dependent effects on the immune balance between PBMC and the tumor cells. The effect of budesonide on tumor development has been examined using animal models. Pereira et al. [32] have shown a dose dependent decrease in multiplicity of lung tumors and their progression to carcinoma in mice treated with various doses of budesonide. Similar observations have been reported by other investigators [33-36]. These reports endorse our results that showed an inhibited cell proliferation with lower concentration of budesonide. Studies, predominant in mice, have shown that the chemopreventive properties of budesonide are generated by various ways, such as modulation of gene expression [37,38] CpG endonuclease activity [39] and remodeling of DNA methylation [40]. The results of the present work revealing the capacity of budesonide to modify the immune cross-talk between PBMC and colon carcinoma cells from the two lines applied in the study suggest the presence of an additional mechanism by which the drug may exert an inhibitory effect on tumor development.
The assistance of Ms. Tzippy Shochat, MSc, Statistical Consultant, Rabin Medical Center, Beilinson Hospital in performing the statistical calculations is highly treasured.