High dose intravenous immunoglobulin therapy (IVIG) has been performed for increase of platelet to patients with immune-thrombocytopenia (ITP). We observed by standard and immunoelectron microscopy the phenomenon of the direct cell to cell contact between red blood cells (RBCs) and endothelial cells (ECs) in bone marrow (BM) of patients with ITP (ITP-patients) after IVIG and then compared to that of the mice injected human immunoglobulin (IgG). RBCs were observed to adhere directly to the ECs of blood vessels in BM of both the ITP-patients and of the mice injected with high dose human IgG. However, in the mice injected with saline, no RBC was seen adhering to the EC as well as that of ITP-patients before IVIG. We concluded that the direct adherence between RBC and EC in the BM after high dosed human IgG injection is not due to an immunological mechanism at all.
IgG, ITP, Human, Mouse, Endothelial cell, RBC, Electron microscopy, High dose immunoglobulin therapy
IVIG: High Dose Intravenous Immunoglobulin Therapy; ITP: Immune-Thrombocytopenia; RBC: Red Blood Cell; EC: Endothelial Cell; IgG: Immunoglobulin; BM: Bone Marrow; ITP: Patient with ITP
IVIG has been used for the temporal increasing of platelet to an ITP-patient [1]. It has been reported that IVIG results in the activation of many immunological functions of the macrophage, dendritic cell or lymphocytes [2-5]. There is also a review about the effect by IVIG to inflammation via IgG glycosylation [6].
We observed the direct cell-to cell contact between macrophages and megakaryocytes [7] by immuno-electron microscopy in BM of an ITP-patient after IVIG and have also found to adhesion between RBC and EC by chance. These pictures had never been observed at the time of BM examination for the diagnosis on admission. Therefore, we have been therewithal aware of a phenomenon in which RBCs were directly linked to the surface of ECs by IVIG. Our previous study gave rise to the postulation that the direct cell-to cell contacts between RBCs and the ECs might be a non-immunological relation elicited by IVIG.
In this study, we injected human IgG product into mice, and then monitored the RBCs and ECs in the vessels of BM by standard- and immunoelectron microscopy to confirm the non-immunological nature of high dose human IgG product serving as an adhesive agent.
Four ITP-patients (2 males and 2 females) were given IVIG (0.4 g/kg given in the course of 5 days). On the final day of IVIG, the BM aspiration was performed on all four patients. The commercial human IgG product (Venoglogulin-I®, Green Cross Co., Japan) was used for the therapy in which the following components were contained in 1 ml; 50 mg of IgG, 10 mg of albumin, 5 mg of polyethylene glycol 4000, 20 mg of D-mannitol and 5 mg of sodium chloride. The morphological observation of megakaryocytes in BM after IVIG of one male or one female patient in this study had been previously reported [7].
Four mice (BALB/c, 2 males and 2 females, 8 weeks old weighing 18~24 g) were intraperitoneally injected the same commercial human IgG product (4 g/kg, in one injection) as that being used in IVIG for patients. BM samples from the mice were collected after the lapse of 24 hours of the injection. As the intravenous injection to mouse was technically difficult for us, human IgG was intraperitoneally administered. The IgG product used for the mice was 10-fold more than the concentration used for the ITP-patients because the intraperitoneal injection would result in lower absorption capacity into BM than intravenous injection. As a control, the equal volume of saline (mL) to the IgG product was injected according to the respective body weight of each mouse.
The immuno-electron microscopic study was performed as previously reported [8]. Briefly, BMs from patients or mice were fixed in a mixture of 0.5% glutaraldehyde, 2% paraformaldehyde and 0.32% picric acid in 0.5M phosphate buffer (pH 7.4) for 30 min and then embedded in Lowicryl K4M (Polysciences). Ultrathin sections made from blocks of Lowicryl K4M were performed immunogold staining method. Polyclonal antibody against anti-human IgG (MBL) was used as the primary antibody, and then 10 nm gold particle-conjugated secondary antibody (GAR G10, Janssen) was added to the ultrathin sections as the secondary antibody. As a control for the staining, human albumin was used as a substitute for the primary antibody.
For the standard-electron microscopy, BM from human or mouse was fixed in 2% glutaraldehyde. 0.1M cacodylate buffer (pH 7.4) for 2 hours and then processed according to the methods as previously reported [9].
In the blood vessels of BMs from ITP-patients, RBCs were observed to contact with the ECs by standard-electron microscopy (not shown). Such ECs were positive for anti-IgG antibody in the cytoplasm and granules. Gold particles were distinctively aggregated along the area of cell-to cell contact between RBC and EC (Figure 1a and Figure 1b). In mice, RBCs and ECs showed the direct cell-to cell contact (Figure 1c and Figure 1d). These pictures declared an exact resemblance to those of RBCs and ECs in BM of ITP-patients after IVIG.
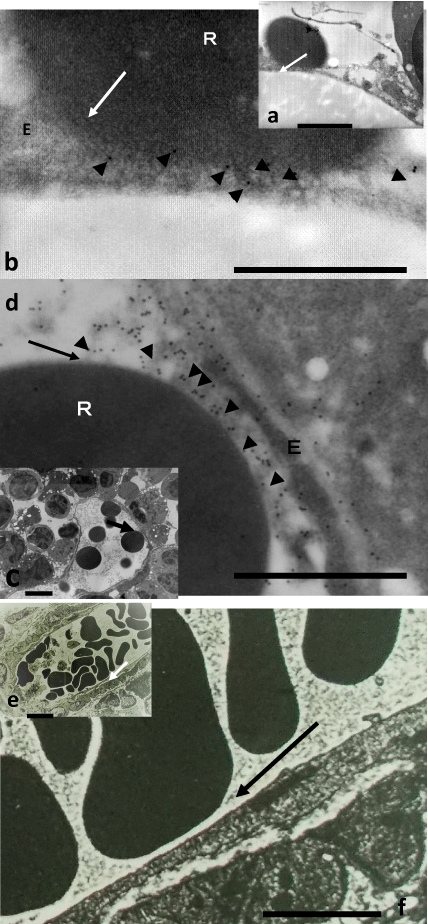 Figure 1: Figure 1b, Figure 1d and Figure 1f (bar = 1 µm) are respectively higher magnification of arrows in Figure 1a, Figure 1c and Figure 1e (bar = 4 µm).
Figure 1: Figure 1b, Figure 1d and Figure 1f (bar = 1 µm) are respectively higher magnification of arrows in Figure 1a, Figure 1c and Figure 1e (bar = 4 µm).
Figure 1a and Figure 1b; human after IVIG, Figure 1c and Figure 1d; mouse after high dosed human IgG product. The membrane of RBC (R) is directly contacted with that of an endothelial cell (E). It is impossible to observe the border of each cell-membrane. IgG (arrowheads) is detected near the cell-to cell contact (arrow).
Figure 1e and Figure 1f; control mouse after saline. The membrane of RBC (R) isn't contacted at all with that of an endothelial cell (E). The membrane of endothelial cell remains imperturbable.
View Figure 1
In controls, no gold particles were observed in the cells by immuno-electron microscopy. Furthermore, in the control study using saline for mice, any RBC wasn't adhered to the EC in BM by standard-electron microscopy (Figure 1e and Figure 1f).
Gold particles, indicating the presence of IgG, were detected in the cytoplasm and granules of ECs in both humans and mice that were administered the commercial human IgG product. This implied that the human IgG product was taken into the ECs of human or mouse. Different from controls, many RBCs directly contacted the membrane of ECs. Furthermore, IgG located near the adhered surface of RBC in the membrane of EC. This indicated that IgG played a very important role with regard to the direct cell-to cell contact between RBC and the EC. Since the same phenomenon was also observed in the BM of mouse after the human IgG injection, it was concluded that the adherence between RBCs and ECs was foreign to any immunological mechanism by IgG. No direct contacts between RBC and the EC were observed in the BMs of the ITP-patients without IVIG on admission (data not shown). In much the same way, any direct adherence between RBC and the EC wasn't observed in the BM of mouse using saline as shown in Figure 1e and Figure 1f by standard-electron microscopy. In our study using mice, the adherence between RBC and the EC was similar to that in BM of human after IVIG. Thus, it was exhibited that the injected human IgG played a role as an adhesive agent between RBCs and the ECs even in the mice. This result suggests that such adhesion of RBCs on the membrane of the EC is a non-immunological phenomenon. This non-immunological phenomenon will be explained as follows; generally, the cell membrane of RBC has a negatively charged potential due to the presence of sialic acid and other related glycoconjugates.
On the other hand, human IgG is positively charged. Because the IgG was detected near the cytoplasmic surface of the ECs in humans after IVIG or in mice after human IgG injection, it showed that the IgG was incorporated into the ECs through its membrane [7]. Thus, it was expected that RBCs came to adhere firmly to the ECs through one of unique electric charge characteristics as the attraction of the positive and negative charges respectively.
The efficacy of IVIG to ITP-patients had been generally attributed immunological mechanisms of IgG [6,10,11]. IVIG has been reported to induce the apoptosis of several cells such as neutrophils or lymphocytes [12]. IVIG has also been proposed to use as one of therapies for recurrent spontaneous abortion (RSA) [13]. Moreover, some reports exist that the degree of efficacy of IVIG in RSA has been reported to be similar to that using albumin [14]. In the experimental study which intraperitoneally injected high dose human albumin to mice, we also observed by electron microscopy that RBCs and ECs were directly contacted each other [15]. Therefore, in this study using mice injected human IgG, we have concluded that the cell-to cell contact between RBCs and ECs might build on a non-immunological mechanism which is similar to the phenomenon using human albumin instead of human IgG [16]. Our experimental results using IgG or albumin seem to be similar to those of clinical trials using IgG or albumin. The cellular contacts among several cells after large amount of IgG or albumin injection might be somewhat related to the effect to RSA after IVIG. Though a small amount of albumin was included in the human IgG product in this study, the lower concentration than that of the previous study [15] was considered to be no concern of the phenomenon between RBCs and the ECs using human IgG product in this study. Thus, our study was considered to indicate that IVIG showed non-immunological function in human body.
Anyway, the clinical significances of the direct cell-to cell contacts by high dosed IgG injection hang in the air. The mechanism or effects of IVIG to cells would be a huge variety. Pharmacological values to several disorders of IVIG should be much more elucidated.