Data on the safety of a cluster regimen to start a subcutaneous immunotherapy (SCIT) with house dust mite (HDM) extract in pediatric patients are scarce. This study investigates the rate of adverse events (AEs) and the applicability of a cluster regimen for HDM SCIT in pediatric patients, and identifies possible risk factors for the development of AEs. A total of 147 injections with a depot extract (D. pteronyssinus / D. farinae, 50% each) were applied to 21 HDM-sensitized pediatric patients (age 5-18 years) with allergic asthma and/or allergic rhinitis within 2 weeks. All patients completed the cluster regimen with the full cummulative dose. Systemic adverse events (AEs) were mild to moderate (rhinitis, nausea or mild asthma), and occurred after 5 injections (3.4%) in three different patients (14.3%). Local AEs occurred in 14 patients (66.7%); 11 patients had minor local AEs and only 3 patients had major local AEs of a diameter ≥ 5 cm. All AEs developed within 60 minutes after the last injection. Patients with local AEs had higher total IgE serum levels than patients without AEs (p = 0.02). In conclusion, the cluster regimen for induction of HDM SCIT is a safe and well tolerated procedure in children.
Dust mite sensitization, Subcutaneous immunotherapy, Cluster regimen, Adverse events, Pediatric patients
Allergen Immunotherapy (AIT) is the repeated administration of allergens to an allergic patient. It is the only disease-modifying therapy for allergies, and prevents the progression of allergic rhinitis to asthma in children [1,2]. In conventional Subcutaneous Immunotherapy (SCIT), allergic patients receive increasing allergen doses every few days during a build-up phase of a few months until a therapeutic maintenance dose, which is then applied every 4-6 weeks. Owing the need of frequent physician consultations and many injections within a short period of time, this build-up phase is inconvenient for patients [3], and the main reason not to start or to discontinue SCIT [4]. Cluster regimens with a few injections on each treatment day provide a faster build-up phase are therefore desirable to improve patient's adherence, and may not necessarily have a higher risk of adverse events (AEs) than conventional updosing regimens [2-5]. Cluster and even rush regimens have been introduced into clinical routine for hymenoptera venom SCIT [6], but are less commonly used for aeroallergens such as house dust mites (HDM) [2] despite a safety profile comparable to conventional SCIT [3,7,8]. Cluster regimens for HDM SCIT have mainly been investigated in adult or mixed adult and pediatric study populations [3,8-12], while data on pediatric populations are rarer [7,13,14]. We therefore carried out this study to assess the safety and tolerability of a cluster regimen for HDM SCIT in a pediatric population, to assess its applicability in the clinical routine of a pediatric hospital, and to identify risk factors for the development of AEs during the cluster regimen.
This is a single-center, retrospective, investigator-initiated study, conducted according to Declaration of Helsinki Principles at the Division of Allergy, Children's Research Center, University Children's Hospital Zurich, Switzerland. All patients were seen between 2006 and 2013 for the duration of the cluster regimen. All patients received the maintenance doses from their pediatrician. The retrospective use of clinical data was approved by the ethical committee of the Canton of Zurich. All patients and and/or legal guardian gave written informed consent for retrospective data analysis. Inclusion criteria were: (i) Age of patient ≤ 18 years; (ii) Sensitization to HDM (Dermatophagoides pteronyssinus and/or D. farinae) as determined by a positive skin prick test and/or detection of allergen-specific serum IgE antibodies; (iii) Presence of allergic asthma and/or allergic perennial rhinitis; (iv) No previous AIT with HDM within the last 12 months and no concurrent AIT with another allergen; (v) Written informed consent signed by the patient and/or legal guardian.
We recorded the following metadata: sex of patients, age at onset of cluster regimen, forced expiratory volume in 1 second (FEV1), mid expiratory flow between 25% and 75% of expired volume (MEF25/75) and fractional exhaled nitric oxide (FeNO). Only FEV1, MEF25/75 and FeNO values determined at day 0 of the cluster regimen before the first injection were included in the analysis. We determined total serum IgE antibodies, and sensitization to perennial and seasonal allergens (D. pteronyssinus and D. farinae, Alternaria alternata, cat dander, dog dander, birch pollen, grass pollen) by skin prick test with standardized allergen extracts (Soluprick SQ, ALK, Volketswil, Switzerland) and/or by detection of allergen-specific serum IgE antibodies using the ImmunoCAP (Thermo Fisher Scientific - Uppsala, Sweden).
All patients received a depot extract adsorbed to calcium phosphate (D. pteronyssinus/D. farinae 50% each; Phostal, Stallergènes, Dietlikon, Switzerland), injected subcutaneously into the dorsal upper arm in a cluster regimen at days 0, 7 and 14 as previously described in adults (Figure 1) [9]. Allergen concentrations in extracts were defined as a reactivity index (IR) of 1 or 10 figure 1, where 100 IR were defined as the allergen concentration eliciting a mean wheal diameter of 7 mm in a skin prick test in 30 HDM-sensitized individuals. For injections at day 0, all patients admitted the intermediate care unit of our hospital, received an i.v. line for security reasons and were monitored for 3 hours after the last injection. Injections at days 7 and 14 were applied at the regular outpatient clinic, and patients were monitored for 60 minutes after the last injection. Local AEs with a diameter < 5 cm were graded as "minor", those with a diameter ≥ 5 cm as "major" [15]. Systemic AEs were graded according the World Allergy Organization [16]. All patients received the maintenance therapy with HDM extract every 4 weeks according manufacturers' instructions from their local pediatrician.
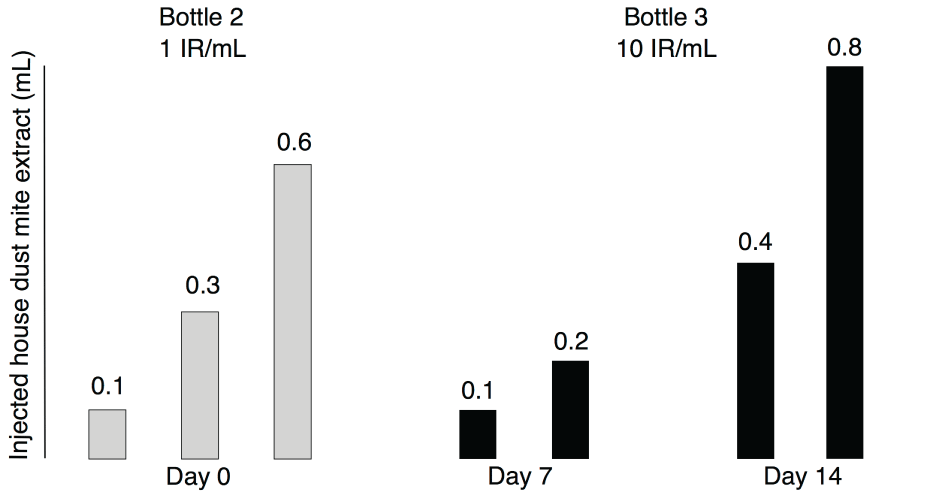 Figure 1: Administration schedule of the cluster regimen. Grey bars indicate injections with house dust mite extract from extract bottle 2 at day 0. Black bars indicate administration of house dust mite extract from extract bottle 3 at days 7 and 14. Height of bars indicate amount of extract injected, the numbers above the bars indicate the injected amount of extract in mL.
View Figure 1
Figure 1: Administration schedule of the cluster regimen. Grey bars indicate injections with house dust mite extract from extract bottle 2 at day 0. Black bars indicate administration of house dust mite extract from extract bottle 3 at days 7 and 14. Height of bars indicate amount of extract injected, the numbers above the bars indicate the injected amount of extract in mL.
View Figure 1
Analysis was performed with R software version 3.2.1 [17]. Outliers of metadata were visualized with Q-Q plots. Continuous metadata were treated non-parametric due to small sample size and presented as median and interquartile range. The rate of AEs in correlation to categorical metadata was tested by Fisher's exact test, in correlation to continuous metadata by Wilcoxon rank test. P values were adjusted for multiple comparison by Benjamini Hochberg correction [18]. Statistical significance for all tests was ascribed to a two-sided alpha level of the adjusted P-values < 0.05, and by 95% confidence intervals for continuous metadata.
Twenty-one pediatric patients were included in this study (Table 1). Twenty patients (95.2%) had allergic asthma. Asthma was well controlled in all patients at onset of the cluster regimen, as defined by the Global Initiative for Asthma [19]. In all of the patients that received a lung function within 1 month before onset of the cluster regimen, the FEV1% was above 80% of the predicted value, but half of the tested patients showed hints to bronchial inflammation (FeNO > 20 ppb). Eighteen of the asthma patients (90%) used inhalation therapies at onset of the cluster regimen. The only patient without asthma was an 18-years-old girl with perennial allergic rhinitis, who therefore qualified for SCIT. All patients were sensitized to either both HDM (n = 20, 95.2%) or to D. pteronyssinus alone (n = 1, 4.8%). Of 16 patients tested for HDM with Immuno CAP (76.2%), 2 patients (12.5%) had HDM-specific IgE antibody serum titers of CAP class 3, 6 patients (37.5%) CAP class 4, 4 patients (25%) CAP class 5, and 4 patients (25%) CAP class 6.
Table 1: Patient demographics and clinical data. View Table 1
All patients completed the cluster regimen and received the full cumulative dose. We applied a total of 147 injections during the cluster regimen. All AEs occurred within 60 minutes after the last injection. The majority of AEs comprised minor local AEs (rash, swelling, pruritus) of < 5 cm in diameter at injection sites, observed in 11 patients (52.4%) (Figure 2).
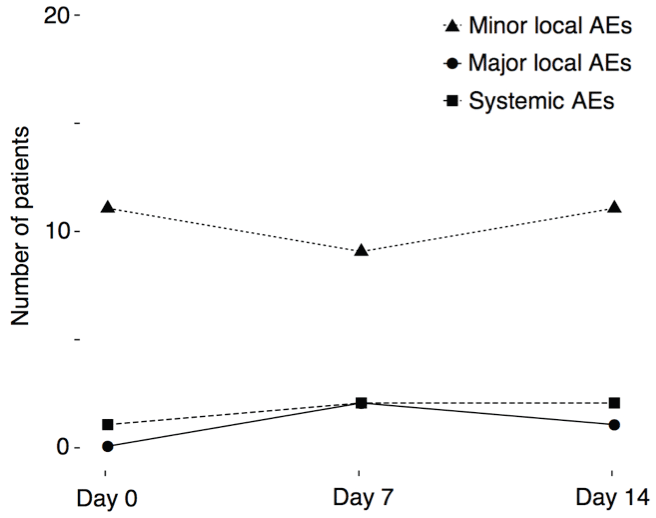 Figure 2: Line plots indicate the number of patients (y-axis) with adverse events during days 0, 7 and 14 (x-axis) of the cluster regimen.
View Figure 2
Figure 2: Line plots indicate the number of patients (y-axis) with adverse events during days 0, 7 and 14 (x-axis) of the cluster regimen.
View Figure 2
Three patients (14.3%) experienced major local AEs of ≥ 5 cm in diameter. Major local AEs did not occur during day 0 of the cluster regimen figure 2, although this was the day with the fastest allergen dose increase and the highest cumulative allergen dose (Figure 1). Two patients had major local AEs at day 7. One was a 15-years-old boy with a 5 × 5 cm red swelling, no AEs at day 0 and a minor local AE at day 14 (8 × 8 mm red swelling). The other patient with a major local AE at day 7 was a 7-years-old girl with a 6 × 6 cm red swelling. She had minor local AEs at day 0 (2 × 2 cm red swelling) and day 14 (8 × 8 mm red swelling). Major local AEs at day 14 occurred in a 12-years-old boy as a 5 × 5 cm swelling at both injections sites, but he had no AEs at days 0 and 7.
Systemic AEs were mild to moderate and observed after 5 injections (3.5%) in 3 patients (14.3%). A synopsis of the patients with systemic AEs is depicted in (Table 2). It is debatable if the malaise of patient 2 (a 7-years-old girl) at day 14 was indeed caused by SCIT. The patient was very nervous before the injections and also developed a viral infection of the upper respiratory tract with sore throat and headache shortly after day 14, both of which may explain the malaise beforehand. She also had minor local AEs with local pruritus at all three days of the cluster regimen. The third patient (an 18-years-old adolescent) who developed a grade II reaction at day 14 had previously received conventional SCIT with HDM for 3.5 years. This SCIT was abrogated 13 months before onset of our cluster regimen due to grade III AEs with urticaria and severe asthma. Owing this history and systemic AEs at the last day of our cluster regimen, he received a halved maintenance dose of 0.4 mL every 4 weeks after the cluster regimen, which was well tolerated during the whole maintenance phase. Notably, only one patient developed a mild (grade I) systemic AEs at day 0 of our cluster regimen, although this was the day with the fastest increase of the allergen doses (Figure 1).
Table 2: Synopsis of patients with systemic adverse events. View Table 2
Patients with mild local AEs tended to have higher total IgE serum levels than children without AEs, but the difference was not significant (p = 0.2). When patients with minor and major local AEs were grouped together, they had significantly higher total IgE serum levels than children without local AEs (p = 0.03, 95% CI [188 - 989]) (Figure 3). This indicates that higher serum levels of total IgE might be a risk factor for the development of local AEs during SCIT with HDM. No other clinical or laboratory metadata, particularly not preexisting asthma, correlated with the development of local or systemic AEs.
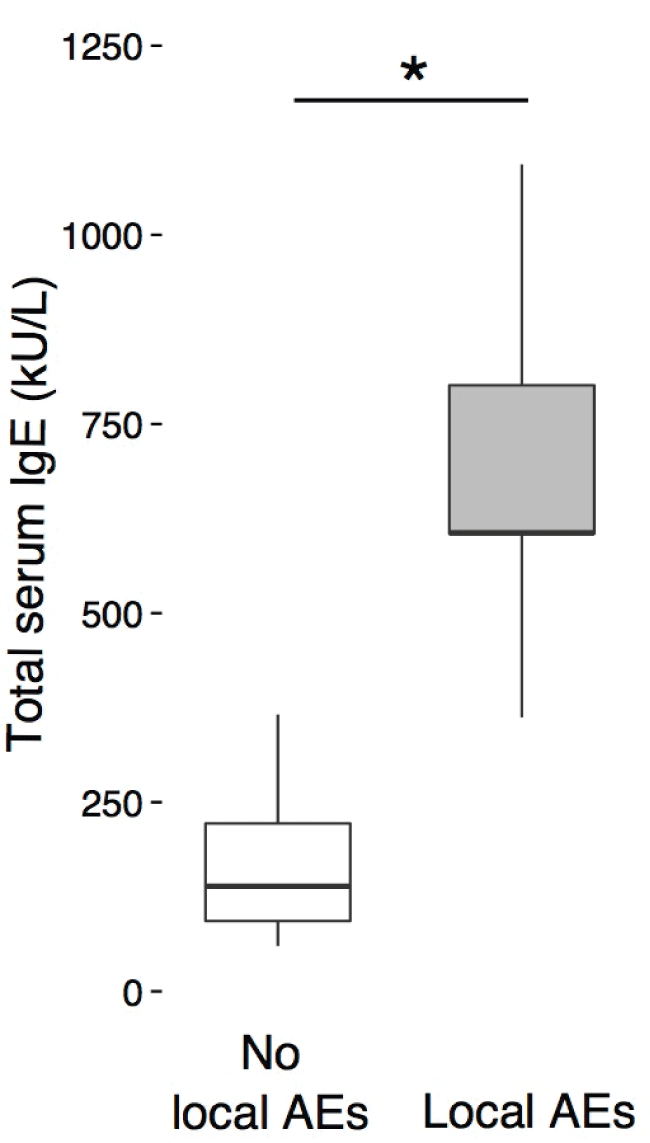 Figure 3: Boxplots indicate total serum IgE levels (y-axis) in patients with local adverse events (AEs) compared to patients with no local AEs.
Figure 3: Boxplots indicate total serum IgE levels (y-axis) in patients with local adverse events (AEs) compared to patients with no local AEs.
*indicates P < 0.05 determined by Wilcoxon rank test.
View Figure 3
This study retrospectively assessed the safety and applicability of a cluster regimen in clinical routine for SCIT with HDM in a pediatric population of 21 patients with allergic asthma and/or allergic rhinitis. In the vast majority, AEs were mild and localized with a red swelling of less than 5 cm in diameter at injection sites, which is considered as common during SCIT and clinically not relevant [1-15]. Systemic AEs and major local AEs were usually mild and occurred in 3 patients (14.3%) each. They were as rare as in adults undergoing the same cluster regimen (15%) [9], resolved with systemic antihistamines or, in one adolescent, with adrenalin inhalation, and did not lead to abrogation of the cluster regimen in any case. These results indicate a comparable rate of local and systemic AEs in pediatric (our study) and adult patients [9], and that it is well tolerated by patients of a wide age range.
In other cluster regimens for HDM SCIT in pediatric and adult populations, between 2.4 and 54.2% of patients had local AEs and between 0 and 3% of patients had systemic AEs [3,8,12-14]. This variability in the rate of AEs may owe to heterogeneity in patient demographics (e.g. age), to different injection schemes, and to different allergen compositions and allergen concentrations of the extracts. For example, a study on 77 children younger than 5 years-of-age [13] reported local and systemic AEs in 3.9% and 1.3% of children, respectively, which appears lower than in our study. However, those children were younger (maximum 5 years) than our patients (maximum 18 years), received only 4 injections (7 injections in our study), and received an allergen extract exclusively containing D. pteronyssinus (D. pteronyssinus/D. farinae 50% in our study) from another manufacturer than in our study. Younger individuals have a less pronounced immune response to certain antigens [20,21] and a lower likelihood for systemic AEs in SCIT than older individuals [22,23]. Also, an extract exclusively containing D. farinae may cause less AEs in HDM sensitized individuals than an extract containing both HDM. Finally, it is difficult to exactly define the allergen concentration of an extract, and it can significantly vary between different manufacturers or even between different batches from the same manufacturer (personal communication with Stallergenes). These factors may contribute to the lower rate of AEs reported by Hernandez et al. [13] than in our study. It therefore appears difficult to compare the rate of AEs between studies using different cluster regimens with different allergen extracts.
The secondary aim of our study was to identify risk factors for the development of AEs during the cluster regimen. We found that higher levels of total IgE in serum are associated with the development of local AEs. A positive correlation between the rate of systemic AEs and serum levels of total and HDM-specific IgE were reported in a Chinese study on 234 HDM-sensitized patients undergoing SCIT [24]. However, these data were published in a Chinese journal and essential information such as on the age of the study population was not available from the English abstract. Positive correlations between the rate of AEs and IgE antibodies have been described for cow's milk-allergic children undergoing oral AIT [25]. However, the significance for our findings remains unclear, because oral AIT in food allergies is usually accompanied by a higher rate of AEs [1].
We acknowledge several limitations of this study. First, this is not a randomized placebo-controlled trial. However, the purpose of our study was not to compare our cluster regimen with other cluster regimens or its efficacy with placebo. The primary aim of this study was to assess the safety and applicability of cluster regimen in the clinical routine and under real life conditions of a pediatric hospital unit. Second, our study population comprised a limited number of patients (n = 21), and the number of patients with major local (n = 3) or systemic AEs (n = 3) was not big enough for a reliable statistical analysis. However, the size of our study population is comparable to previously published studies on HDM cluster regimens, comprising 15-29 patients [3,7,8,11]. Because the cluster regimen used in our study has already been assessed for its safety and applicability in 47 adult patients [9] and the rate of AEs in pediatric and adult patients is similar, the size of our study population appears sufficient. Some other studies on HDM cluster regimens included a higher number of patients (n = 40-343) [8,10,12,14], but comprised a more heterogeneous study population of pediatric and adult patients and did not adjust statistical analyzes for different age groups [8,10,12], or for different cluster regimens [14].
The cluster regimen used in our study is advantageous particularly for pediatric patients. It comprises only 7 injections within 2 weeks and therefore needs fewer injections and is faster than most other regimens ranging from 8 injections during 3 weeks to 16 injections during 6 weeks [3,7,8,10-12,26]. A fast cluster regimen with few injections is desirable for children and their parents. In our experience, parents usually choose the faster cluster regimen, because it requires less physician visits and therefore keeps their absences of work and those of their children of school to a minimum. Also, young patients usually do not like many injections. Hence, the cluster regimen introduced here might improve adherence of pediatric patients and their parents to SCIT. Adherence to SCIT is of particular importance, because type 1 regulatory T cells, which are crucial for the development of allergen tolerance, increase in the blood of pediatric patients (8-13 years) after 1 year of HDM SCIT [27].
In conclusion, the cluster regimen presented in this study seems to be safe for pediatric patients with allergic asthma and rhinitis, and well applicable in clinical routine. AEs during this cluster regimen for updosing HDM SCIT were usually localized and mild. High total IgE serum levels might indicate a higher risk for local AEs. Because patients reach the maintenance phase of SCIT faster than with other cluster regimens, it may be a useful approach to improve also the adherence of patients to treatment.
The retrospective assessment and analysis of clinical data was approved by the ethical committee of the Canton of Zurich.