Chemotherapy schedule has been reported to increase the risk of suboptimal outcomes among cancer patients receiving chemotherapy with variying outcome between treatment modality. This study investigates the Overall Survival (OS) and Hazard of Death (HR) of breast cancer patients with chemotherapy schedule modification stratified against adjuvant (ACT) and neoadjuvant (NACT) treatment modalities. The data required for this study was extracted from hospital based registry. Those patients included in the study were adult female patients receiving chemotherapy between 2013 and 2017 and completed all six chemotherapy cycles. Patients who completed all cycle with a cumulative length of delay < 7 days was categorized as 'no schedule modification' and patients who completed all cycle with the cumulative length of delay ≥ 7 days were categorized as 'with schedule modification'. The Kaplan-Meier estimator was used to estimate survival curves for each covariate and the log rank test was used to evaluate the differences in survival times for each category. Among 124 patients included in the study,93 patients were censoredand 31 events was observed, providing an OS of 75.0% with a mean survival of 54.09 months (95% CI 49.36-58.83). There was significantly higher survival (p < 0.001) in ACT treatment (83.9%) and higher mortality in NACT treatment modalities (51.6%). Among ACT treatment modality, those with no schedule modification had a significant proportion of patients surviving(p = 0.04) compared to patients with schedule modification . The OS was significantly different between age (p = 0.013), stage (p = 0.022) and chemotherapy (p = 0.002) and no significant difference in the distribution in NACT modality. Our finding suggests that patients with advanced-stage might have better survival implications when the chemotherapy schedule is optimized. Thus, the risk versus benefit of schedule modification must be carefully managed to ensure optimal chemotherapeutic outcomes while balancing the concurrent toxicity.
Breast cancer continues to be a commonly diagnosed cancer among Malaysian women, with annual increases in the number of diagnoses [1]. The Malaysian National Cancer Registry (NCR) reported that 18,343 new breast cancer diagnoses were diagnosed from 2007 to 2011, accounting for 17.8% of total cancer cases [2], while for the year 2018 alone, it is estimated that 7593 new breast cancer cases were reported [3].
Although the worldwide breast cancer mortality rates have been declining over the decades [4], the Malaysian breast cancer mortality rate was still high at 11.82% [2] compared to the Asian average of 6.05% [5]. Regionally, Malaysia has a breast cancer mortality rate of 18 per 100,000 while Singapore and Thailand with 15 and 11 per 100,000 populations, respectively [6]. Likewise, the overall survival (OS) of Malaysian breast cancer survival is at 66.8%, which is lower than in neighbouring Asian countries, such as 70% achieved by Singapore [7] and ≥ 80 % survival achieved by Korea (91.2%) [8], Japan (88.1%) and China (82%) [9].
The low OS among Malaysian breast cancer patients is worrying as Malaysian breast cancer patients can seek cancer diagnosis and treatment in either government-funded or self-funded health care systems. In addition, Malaysia's healthcare system is highly accessible, with 92% of urban and 69% of rural populations have access to healthcare facilities [10]. The low OS raises the need to evaluate treatment-related variables, which could identify factors that negatively impact the survival outcome. While there have been numerous studies of prognostic factors among Malaysian breast cancer patients, these studies are generally limited to non-modifiable traditional prognostic factors such as demographic and disease-related prognostic factors.
Patients prognoses are known to be dependent on the treatment modalities. Once the patients are diagnosed with breast cancer, the spread of the disease determines if adjuvant chemotherapy (ACT) or neoadjuvant chemotherapy (NACT) treatment modality is indicated. The use of ACT is responsible for reducing global breast cancer mortality. The 20-year follow-up of The Early Breast Cancer Trialists' Collaborative Category meta-analysis published in 2012 reported ACT decreased breast cancer mortality from 36% to 29 % (HR 0.79; 95% CI 0.72 to 0.85) when adjusted for nodal status, tumour size or grade and Estrogen Receptor (ER) status [11]. NACT is generally used to render inoperable breast cancer resectable, decrease axillary lymph node dissection, and evaluate tumour response to chemotherapy [12]. The safety and survival outcomes of NACT have been studied in several randomized trials [12-15]. The National Surgical ACT Breast and Bowel Project (NSABP) Protocol B-18 study reported comparable OS between ACT and NACT modality over 16 years of follow-up [16]. However,meta-analysis indicates no difference in OS benefit (HR 1.03; 95% CI 0.94 to 1.13, p = 0 .51) between the ACT and NACT modality categories [12]. Despite the benefit of NACT, a higher risk of local recurrences and a decrease in OS was reported for NACT [17]. While the available evidence indicates s similar OS rates between NACT and ACT for operable breast cancer, the OS among inoperable breast cancer remains questionable.
The principal goal of both this treatment modality is to improve patient outcomes by addition on chemotherapy over what can be obtained from surgery alone. However, the chemotherapy schedule is often modified in clinical practice due to treatment-related toxicity, contributing to delays in completing the chemotherapy regimen. Recently there have been increased interest in chemotherapy schedule as a clinically modifiable prognostic factor. Prolonged schedule modification has been reported to increase the risk of suboptimal outcomes among cancer patients receiving chemotherapy [18,19]. Hariyanayagam, et al. have reported that in public hospitals, very few patients (16.4%) were able to complete chemotherapy with no schedule modification, while the majority of the patients (69.0%) experienced schedule modification and some patients (14.6%) had an incomplete schedule [20]. Among this cohort of patients, those with schedule modification had a 2.34-times higher risk of death (Hazard Ratio (HR) 2.34, 95% CI 1.03–5.32; p = 0.043) compared to patients with no schedule modification. Similarly, Liutkauskiene, et al. reported that patients who experienced chemotherapy schedule modification had a 3.3-times higher risk of death than patients who did not experience any chemotherapy schedule modifications (HR3.3, 95% CI 1.2–8.5, p = 0.016) [19]. Several other studies have reported a similar observation of lower survival rates among patients who experienced schedule modification [18-24].
There is a general lack of information on chemotherapy schedule modification experienced by breast cancer patients in Malaysia. This study investigates the OS and HR of breast cancer patients with chemotherapy schedule modification stratified against ACT and NACT treatment modalities. Understanding the impact of chemotherapy schedule modification within treatment modalities could explain the low OS in this region.
Hospital-based registry data from the Oncology Pharmacy department, Hospital Seri Manjung, was used to extract the required information for this study. Those patients included in the study were adult female patients receiving chemotherapy between 2013 and 2017 and completed all six chemotherapy cycles. Patients with incomplete medical record (n = 4), regimen changes during chemotherapy (n = 5), nonstandard chemotherapy regimen (n = 4), on palliative care (n = 12) or transferred between facilities (n = 4) was excluded. The ethical approval to conduct the study was obtained from the Medical Ethical Review Committee [MERC KKM. NIHSEC. P18-1872 (6) Date 27-09-2018], Ministry of Health, Malaysia. The review board exempted the need for informed consent to conduct this study.
Prognostic factors under investigation for this were extracted from the registry. The demographic and clinical characteristic includes age at chemotherapy (< 50 or ≥ 50 years), ethnicity (Malay or Non-Malay), pathological stage according to the 6th edition of TNM classification by American Joint Committee on Cancer (AJCC) [Early stage (Stage I and II) or Advanced stage (Stage III and IV)] [25], molecular subtypes [Luminal A (ER-positive/PR-positive, HER2-positive), TNBC (ER-negative, PR-negative, and HER2-negative) and other types (ER-positive/PR-positive, HER2-negative and ER-negative, PR-negative, and HER2-positive)] [26], treatment modalities (adjuvant or neoadjuvant), chemotherapy regimen (Anthracycline-based, taxane-based or Anthracycline + Taxane) [20]. The length of the chemotherapy regimen was calculated from the first date to the last date of chemotherapy administration. Patients who completed all cycle with a cumulative length of delay < 7 days was categorized as 'no schedule modification' and patients who completed all cycle with the cumulative length of delay ≥ 7 days were categorized as 'with schedule modification'.
The primary outcome of interest was overall survival stratified against treatment modalities, with time from administration of chemotherapy as time scale (month). The patients status (alive or dead) on 31 Dec 2018 was verified with the National Registry Department, Malaysia, through the Institute of Clinical Research, Malaysia.
All categorical variables were presented as the number (n) and percentage (%). Patient's demographic and clinical characteristic association between chemotherapy schedule modification were evaluated using the Chi-square test. The mortality rate was calculated for schedule modification category stratified for treatment modalities to evaluate chemotherapy schedule modification impact on survival outcome (alive or dead).
The Kaplan-Meier estimator was used to estimate survival curves for each covariate. Time in this study is defined as the duration in the month from the first date of chemotherapy until the date of event or censor. The rate of those who had the event and censored is reported using frequencies and percentages for each covariate. The mean survival time with a 95% confidence interval was reported as more than 50% of study participants are still alive at censoring. A Kaplan-Meier survival curve was generated to display survival curves of the cumulative probability of an individual remaining alive during a unit of time (month). The log rank test investigates the observed and expected number of differences in survival times for each category. For covariates with significant survival distribution, a pairwise comparison over strata was made to test the equality of survival distribution within the covariates. Multivariate analysis using Cox regression was conducted with all covariates. The factor levels of each covariate were compared to the first category. To evaluate the effects of individual predictors, Exp (β), the Hazard Ratio (HR), was interpreted as the predicted change in the hazard for a unit increase in the predictor. Adjusted Hazard Ratio (AHR) above 1 indicates covariates have higher event probability, thus negatively affecting the length of survival. HR was presented with a 95% confidence interval. The p-value (sig) < 0.05 is considered to significantly differ in HR between the category in a covariate. Descriptive and inferential analysis of the data was performed using Statistical Package for the Social Science (SPSS) for Windows version 15.0 (SPSS Inc., Chicago, Illinois, USA).
A total of 124 female breast cancer patients were included in the study (Table 1). The majority of the pateints received adjuvant treatment modalities (n = 93 , 75%), and the remaining received NACT treatment modalities (n = 31, 25.0%). Between both treatment modalities group , majority of the patients were more than 50 years old [(adjuvant; n = 62 , 66.7%) , (NACT ; n = 17 , 54.8%)], malay ethnic [(ACT; n = 54 , n = 58.1%) , (NACT ; n = 24 , 77.4%)] , had advanced stage disease [(ACT; n =`54 , 58.1%), (NACT; n = 26 , 83.9%)], with other types of molecular subtype [(ACT; n = 44, 44.7%), (NACT; n = 20 , 64.5%)] and received anthracycline based chemotherapy [(ACT; n = 57, 61.3%), (NACT ; n = 23,74.2 %)]. interestingly, both treatment modalities had similar propotion of schedule modification [(ACT ; n = 52, 55.9%), (NACT; n = 18, 58.1%)]. The propotion of patients surviving was higher in ACT modality compared to NACT modalities, [(ACT; n = 78, 83.9% ), (NACT; n = 15, 48.4 %)].
Table 1: Demographic and clinical characteristicsaccording to treatment modalities (n =124). View Table 1
There was a significantly (p = 0.009) difference in early-stage (41.9% vs. 16.1%) and advanced stage (58.1% vs. 83.9%) between ACT treatment and NACT treatment modality. Similarly, there was significantly higher survival (p < 0.001) in ACT treatment (83.9% vs. 48.4%) and higher mortality in NACT treatment modalities (51.6% vs. 16.1%).
As for treatment outcome (Table 2), 93 patients were censored (alive), and 31 events (death) was observed, providing an overall survival of 75.0% with a mean survival of 54.09 months (95% CI 49.36-58.83). Among patients receiving ACT treatment modality, those patients with no schedule modification (92.7%) had a significant proportion of patients surviving(p = 0.04) compared to patients with schedule modification (76.9%). Among patients receiving ACT treatment modalities, there was a significantly higher survival rate among patients < 50 years old (p = 0.013), stage I/II (p = 0.022) and type of chemotherapy (p = 0.002). The survival outcome among patients receiving Anthracycline (80.7%) and Anthracycline + taxane combination (93.9%) was superior to Taxane-based chemotherapy.
Table 2: The survival outcome between schedule modification and treatment modality (n = 124). View Table 2
Table 3 presents the overall survival for all covariates between the treatment modalities. The OS was significantly different between age (p = 0.013), stage (p = 0.022) and chemotherapy (p = 0.002) among ACT modality and no significant difference in the distribution in NACT modality. In addition, the OS among ACT patients (Figure 1) with no schedule modification (92.7%) was higher than those with schedule modification (76.9%). However, the difference in survival distribution was not significant (p = 0.058). On the other hand, among patients in NACT treatment modality (Figure 2), the survival difference among patients with no schedule modification (46.2%) was lower than those with schedule modification (50.0%). Similarly, the difference in survival distribution was not significant (p = 0.883)
Table 3: Overall survival according to demographic and clinical characteristic stratified against treatment modalities (n = 124). View Table 3
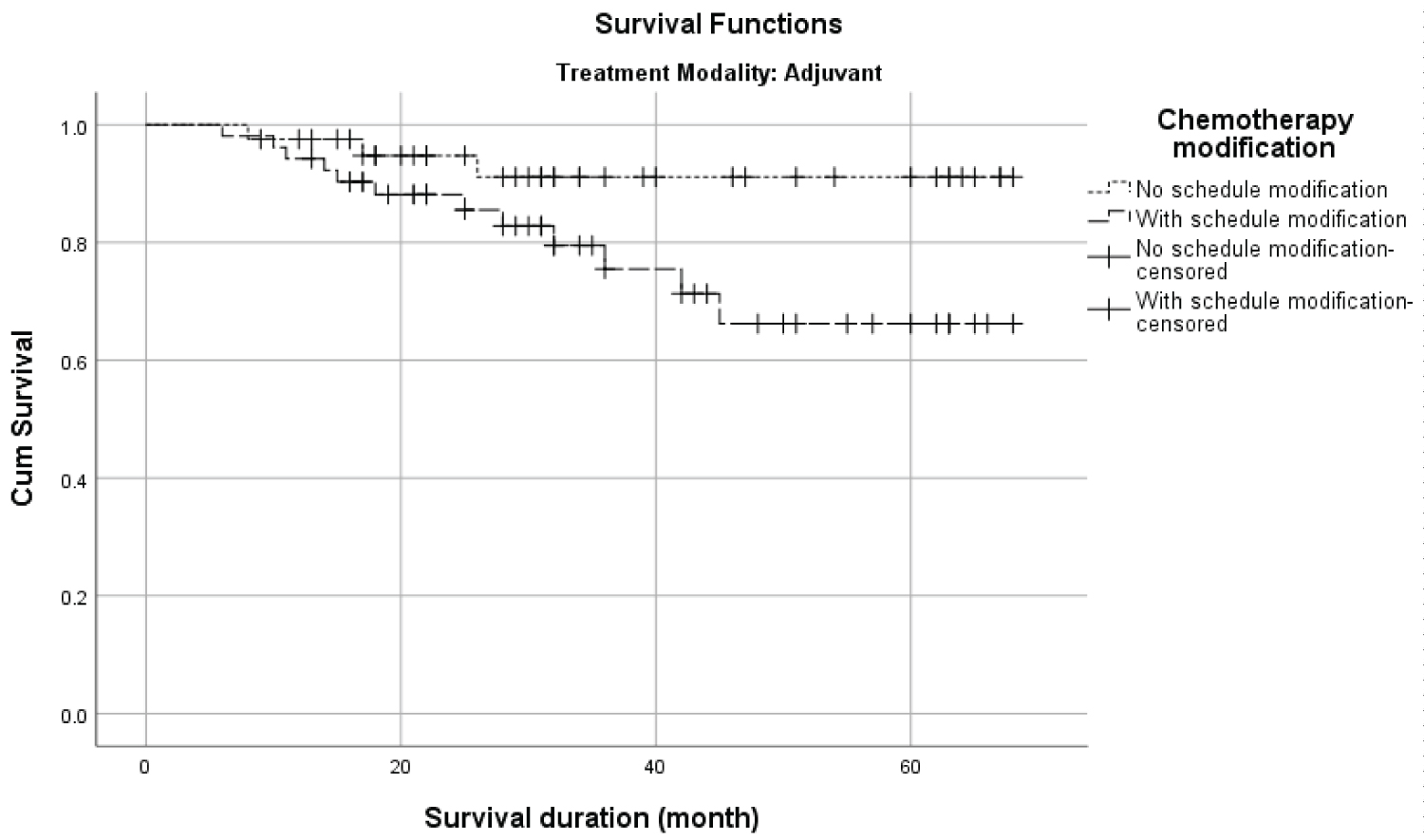 Figure 1: The Kaplan-Meier survival distribution of chemotherapy schedule modification in adjuvant treatment modality.
View Figure 1
Figure 1: The Kaplan-Meier survival distribution of chemotherapy schedule modification in adjuvant treatment modality.
View Figure 1
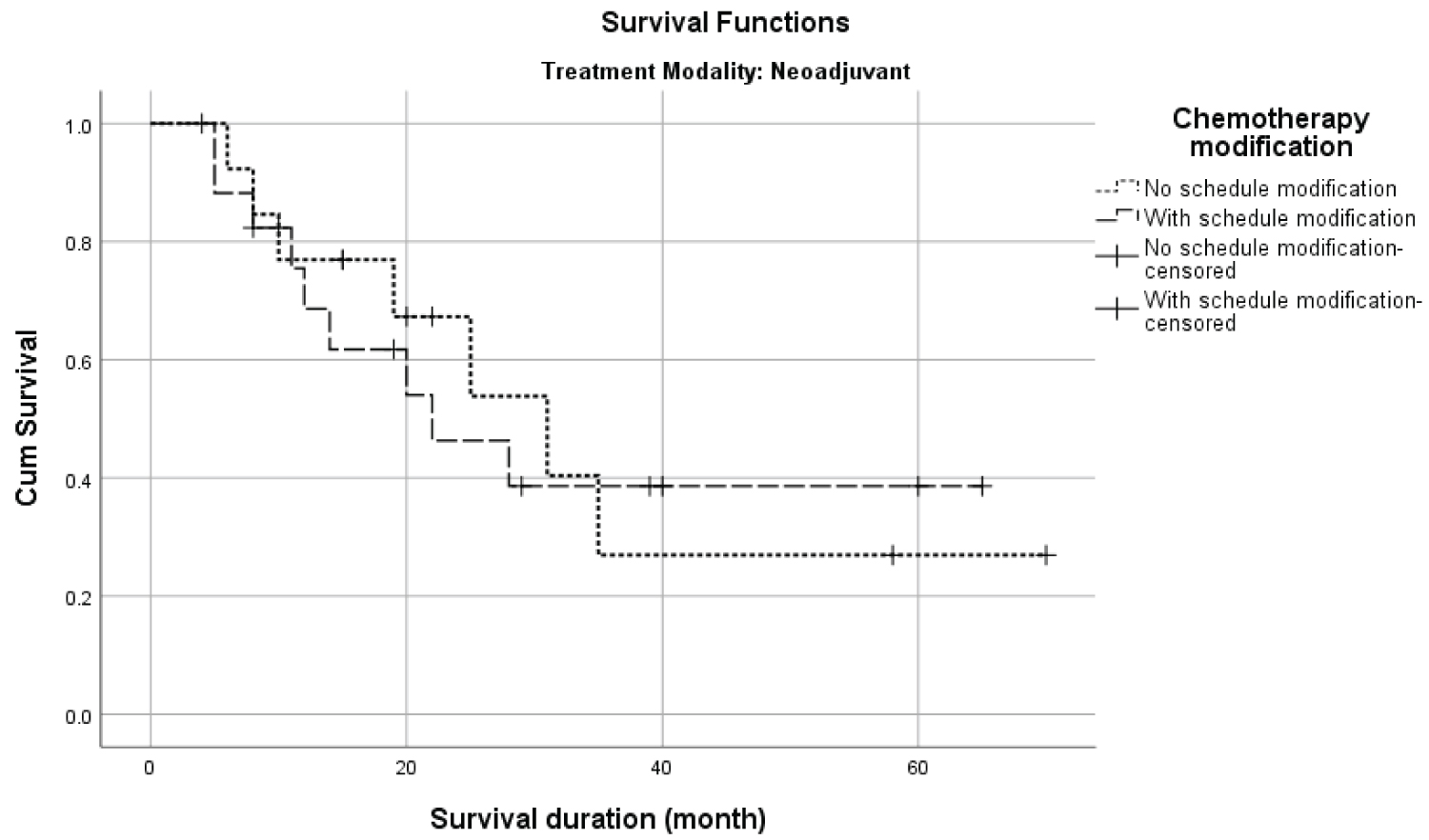 Figure 2: The Kaplan-Meier survival distribution of chemotherapy schedule modification in neoadjuvant treatment modality.
View Figure 2
Figure 2: The Kaplan-Meier survival distribution of chemotherapy schedule modification in neoadjuvant treatment modality.
View Figure 2
A multivariate Cox proportional hazard regression model for OS adjusted for all the investigated covariates was performed for both ACT and NACT treatment modalities (Table 4). Among patients in ACT modality, advanced stage patients had risk of death increased by 11.16 times (AHR 11.16, 95% CI 2.15-37.90 ; p = 0.04) compared to early stage. Patients who were receiving Taxane based chemotherapy had risk of death increased by 6.15 times (AHR 6.15, 95% CI 1.38-10.54; p = 0.022)while those who were receiving Anthracycline + taxane combination had hazard of death reduced by 0.21 times (AHR 0.21, 95% CI 0.04-1.04; p = 0.49) when compared to Anthracycline based chemotherapy regimen. There was no notable increase in the hazard of death among NACT treatment modality among all investigated covariates.
Table 4: Prognostic factors of death by multiple cox proportional hazard regression (n = 124). View Table 4
We evaluated the distribution and survival outcome of various covariates with treatment modalities. Our results demonstrated that patient distribution between the covariates was independent of treatment modalities except for cancer stage and survival outcome. Our study observed a high proportion of patients presenting at an advanced stage between treatment modalities similar to the previous decade [27,28], highlighting late diagnosis as an unmet public health concern. However, we only included patients receiving six cycles of chemotherapy regimen, which generally consist of patients with advanced disease, explaining the high proportion of advanced stage in our study.
The majority of patients received ACT as ACT modality are known to provide a reduction of up to15% in breast cancer mortality [29]. In Malaysia, ACT has been the mainstay treatment modality as up to 80.5%-90.1% will receive ACT treatment modality. However, the lower proportion of ACT observed in this study was due to the inclusion of advanced stage breast cancer, which has been largely excluded from other studies [30-32]. On the other hand, NACT is preferred to downstage locally advanced disease and make it operable, particularly for large tumours [33]. The lower OS among NACT could be correlated with the use of this modality to treat patients with locally advanced breast cancer [34,35]. While NACT has been reported to increase tumour response rates and breast-conserving therapy [36], NACT has not demonstrated improved survival over ACT chemotherapy in randomized trials [17].
Patients who completed chemotherapy with no schedule modification had significantly higher survival compared to patients with schedule modification. Deviation from the chemotherapy schedule might account for increase in mortality for the advanced stage. Subgroup analysis indicates that patients with no schedule modification had a significantly higher proportion of patients surviving than patients with schedule modification. Our result suggests that chemotherapy schedule modification contributed to sub-optimal outcomes, further supporting previous studies that correlated schedule modification with suboptimal antitumor efficacy and reduced survival rates [18,19,21-24,37,38]. The negative survival impact could be potentiated by increased duration between chemotherapy cycles, reducing the treatment's dose intensity. Henderson et al. proposed that the anti-tumour activity of cytotoxic agents depends on the drug used and on the schedule of drug administration [39]. This observation was further demonstrated in 20 years of a follow-up study by Bonadonna, et al. which reported a negative association between reduced delivered dose and survival outcome [40].
Interestingly, among patients with no schedule modification, the OS was higher (92.7%) for ACT treatment modality and lower (46.2%) for NACT modality. Our observation highlights the importance of adhering to the chemotherapy schedule to ensure adequate dose intensity according to the trial protocol. Dose intensity of administrating chemotherapy once every three weeks provided the optimal treatment benefit against disease progression. An Australian-based study has observed a reduction in drug intensity associated with lower OS, even when adjustment to other prognostic parameters [41]. Similarly, low dose intensity has been correlated with poor tumour growth control, reduced quality of life and decreased overall survival among ACT patients [15,42].
As for NACT, the rescheduling of chemotherapy due to treatment toxicity is an indicator of adequate chemotherapeutic dosing. The histological tumour regression provides a better clinical outcome among preoperative patients. A study by Wu, et al. has reported that treatment toxicity was associated with better disease-free survival (P = 0.116) and superior OS (HR 0.25, 95% CI: 0.077–0.830, P = 0.023) [43]. Similarly, many studies have reported the association between treatment toxicity and better clinical outcomes, and our study corroborated their findings [44,45]. However, when adjusted for all other covariates, chemotherapy schedule modification was not a significant predictor of death in both treatment modalities.
Among patients in ACT modality, advanced stage patients had risk of death increased by 11.16 times (AHR 11.16, 95% CI 2.15-37.90 ; p = 0.04) compared to early stage. Our finding is similar to the NCR report of unadjusted HR of 7.52 (95% CI 6.83-8.28) for advanced stage compared to early stage [2]. Our finding confirms that pathological stages are the most important prognostic factor even when adjusted for other confounding factors.
Antracycline+Taxane combination regimen has substantially improved outcomes in breast cancer patients [46] and reduced mortality rates compared to anthracycline-based regimens alone [47]. This is evident from our study where patients receiving Antracycline+Taxane combination chemotherapy HR reduced by 0.21 times (HR 0.21, 95% CI 0.04-1.04; p = 0.49) compared with patients receiving Anthracycline based chemotherapy. The survival benefit observed in our study is similar to a recently published meta-analysis of 29 studies involving 41,911 women, which showed Antracycline+Taxane combination chemotherapy improved OS compared to chemotherapy without the combination (HR 0.87; 95% CI 0.83 to 0.92) [48]. Our finding supports the wider use of Antracycline+Taxane combination chemotherapy regimens for the observed additional clinical benefit. Patients receiving a Taxane-based regimen were associated with increased HR by 6.15 times (AHR 6.15, 95% CI 1.38-10.54; p = 0.022). The increased HR was expected as Taxane based regimen is usually given for advancedstage patients who had an inherently poor prognosis due to the disease spread. Despite this, Taxane is known for providing a response rate of 50% to 68%, although the duration of response is usually short [49-51]. For this reason, Taxane is indicated as a first-line treatment for advanced stage, even if with a short serving benefit.
Our finding suggests that patients with advanced-stage might have better survival implications when the chemotherapy schedule is optimized. Several large institutional series described prolonged survival in women with advanced disease treated with chemotherapy therapy [52,53]. Deviation from the chemotherapy schedule might account for increased HR for the advanced stage. Although we found no clear association of increased hazard risk of chemotherapy schedule modification when adjusted for other covariates. The reduction in overall survival and the increased risk of death, albeit the non-significance, calls for more studies for this modifiable covariate.
This study reflects the chemotherapy schedule as a shortfall in cancer patient management. Our result suggests that deviation from the planned chemotherapy schedule could be associated with poorer outcomes. Thus, the risk versus benefit of schedule modification must be carefully managed to ensure optimal chemotherapeutic outcomes while balancing the concurrent toxicity.