This article describes a SEER database study of 1,595 patients with adenoid cystic carcinoma of the head and neck. Surgery was associated with improved survival, while adjuvant radiotherapy showed no significant improvement in overall survival.
The effect of surgical resection (SR) and/or adjuvant radiotherapy (RT) on overall survival (OS) for patients with non-metastatic adenoid cystic carcinoma (ACC) of the head and neck has not been clearly established.
The primary endpoint was overall survival (OS). Univariate and multivariate analyses were performed on pretreatment clinical variables.
The records for 1,595 patients with non-metastatic ACC of the head and neck were obtained from the SEER database. Multivariate analysis revealed that younger age at diagnosis, surgery performed, gross resection type, no lymph node involvement, smaller tumor size, and major salivary gland and palate primary tumor sites to be prognostic for a statistically improved OS (p < 0.001). The addition of adjuvant RT was not associated with an improvement in OS for patients.
This study reveals that age of diagnosis, absence of lymph node involvement, smaller tumor size, and extent of surgery were positive predictors for statistically significant improvements in OS in patients with ACC of the head and neck. Further clinical studies will be needed to further elucidate the impact of surgical resection type and adjuvant RT techniques on local regional control and risk of distant metastases.
Adenoid cystic carcinoma, Head and neck, Surgery, Adjuvant radiation, Observation
Adenoid cystic carcinoma (ACC) is an uncommon histology that develops from major and minor salivary gland tissue. ACC can originate from multiple different organs but is primarily associated with the salivary gland tissue in the head and neck. According to He S, et al., ACC follows an indolent clinical pattern with a tendency for perineural invasion and accounts for three to five percent of all head and neck cancers [1]. Sayan M, et al. "estimates the global incidence rate to be 0.4 to 13.5 cases per 100,000 annually" [2]. According to the International Head and Neck Scientific Group, the parotid gland is the most frequent location of primary salivary gland ACC [3]. Cao C, et al. found that the most common location site of minor salivary gland ACC is the oral cavity followed by the paranasal sinuses [4]. ACC can affect all ages of life, but most commonly affects individuals in their 50s and 60s and there are no known risk factors [4].
Historically, the mainstay treatment for ACC has been surgical resection of the primary tumor followed by adjuvant radiotherapy (RT) [2]. However, the lack of data regarding ACC treatment has made it difficult to solidify a widely accepted treatment protocol. In some clinical settings, patients undergo elective neck dissection, but there is debate regarding its benefit. According to the International Head and Neck Scientific Group, the prevalence of cervical lymph node metastasis in ACC is an uncommon finding and suggests that elective neck dissection may not be necessary [3,5]. There is also debate over how beneficial adjuvant RT is on overall survival (OS), but research by Sayen, et al. indicates that it may improve overall local regional control of disease [2].
The purpose of this paper is to assess the effect of surgery and adjuvant RT on the OS of non-metastatic ACC of the head and neck using the Surveillance, Epidemiology, and End Results (SEER) database. The abstract of this paper was presented at the ninth American Head and Neck Society International Conference in Seattle, WA [6].
All data for this study were obtained from the National Cancer Institute's Surveillance, Epidemiology, and End Result (SEER) database. The SEER database compiles data from 20 regional registries in the United States, covering approximately 28% of the U.S. population, which are operated by non-profit organizations under contract with the National Cancer Institute (NCI). These regional registries include the Alaska Native Tumor Registry, Arizona Indians, Cherokee Nation, Connecticut, Detroit, Atlanta, Great Georgia, Rural Georgia, San Francisco-Oakland, San Jose-Monterey, Greater California, Hawaii, Iowa, Kentucky, Los Angeles, Louisiana, and New Jersey. The SEER database is updated on an annual basis. Before data is entered into the registries, all patient identifiers are removed in order to preserve patient confidentiality. To insure accuracy and quality of the database, the NCI conducts routine reviews and updates the database when necessary. Because the SEER database is available to the public, Institutional Review Board approval was not required to perform this analysis.
Data from 1,595 patients with non-metastatic adenoid cystic carcinoma of the head and neck were collected from the SEER database between 1973 and 2012. The following clinical variables were collected from the 1,595 patients: age at diagnosis (< 40, 40-59, 60-79, 80+); race (White, Black, Pacific Islander/Asian, or unknown); gender; primary site of origin (major salivary gland, palate, unspecified mouth part, tongue, mouth floor, nasopharynx, lip, or other); tumor size (< 21 mm, 21-40 mm, > 40 mm, or unknown); lymph node involvement (yes, no, unknown); surgery (yes, no, or unknown); extent of surgery (gross, partial, or none); and addition of adjuvant RT (yes, no, or unknown). Gross resection is defined by the SEER database as total resection of the primary site. Partial resection is defined as partial resection of the primary site. Patients with previous malignancy, distant disease, incomplete or conflicting records, and/or atypical histologic features were excluded from this analysis.
Survival curves were estimated using the Kaplan-Meier method and used to compare all of the clinical variables. Andersen 95% confidence intervals for the median survival time of the clinical variables were created. Log-rank tests were performed to determine if there was statistical evidence of differences among the survival curves of the above clinical variables. Finally, the Cox proportional hazard model was used in a multivariate analysis of the clinical variables.
A total of 1,595 patients met the inclusion criteria for the study. Patient demographics and clinical characteristics are shown in Table 1. Of the 1,595 patients included in the study, 242 were under the age of 40, 687 were between the ages of 40-59, 543 were between the ages of 60-79, and 123 were over the age of 80. One thousand, one hundred and sixteen patients received RT, 454 did not, and 25 were of unknown status. Five hundred and thirty-five patients received surgery, 62 did not, and 998 were of unknown status. Of those that received surgery, 383 underwent gross surgery, 148 underwent partial surgery, and four underwent an unknown surgery type. Three hundred and sixty-seven patients received both surgery and post-operative radiation.
Positive lymph node involvement was reported in 392 patients. One thousand, one hundred and ninety-four patients had no lymph node involvement, and nine were of unknown status. In 935 patients, the primary tumor site was the major salivary glands, 266 patients had a tumor of the palate, 111 had a tumor of unspecified mouth parts, 108 had a tumor of the tongue, 43 of the mouth floor, 53 of the nasopharynx, 49 of the lip, and 30 of other locations.
Table 1: Patient and treatment characteristics. View Table 1
Univariate analysis of each subgroup is shown in Table 2. Median OS in the < 40 age was incalculable as more than 50% of patients survived by the end of the study. Median OS in the 40-59, 60-79, and 80+ age groups were 248, 122, and 58 months, respectively. Using the < 40 age group as a reference, 95% confidence interval hazard ratios revealed statistically inferior outcomes in the older groups with a P < 0.001 in all cases as demonstrated in Figure 1. Analysis revealed that males have a statistically inferior outcome compared to their female counterparts as indicated in Figure 2 (HR: 1.24 [95% CI, 1.06-1.46], P = 0.008). Comparison of median OS amongst White, Black, Asian/Pacific Islander, and Other/Unknown races revealed no statistically significant differences with the following P values with respect to race: P = 0.999; P = 0.055; P = 0.323.
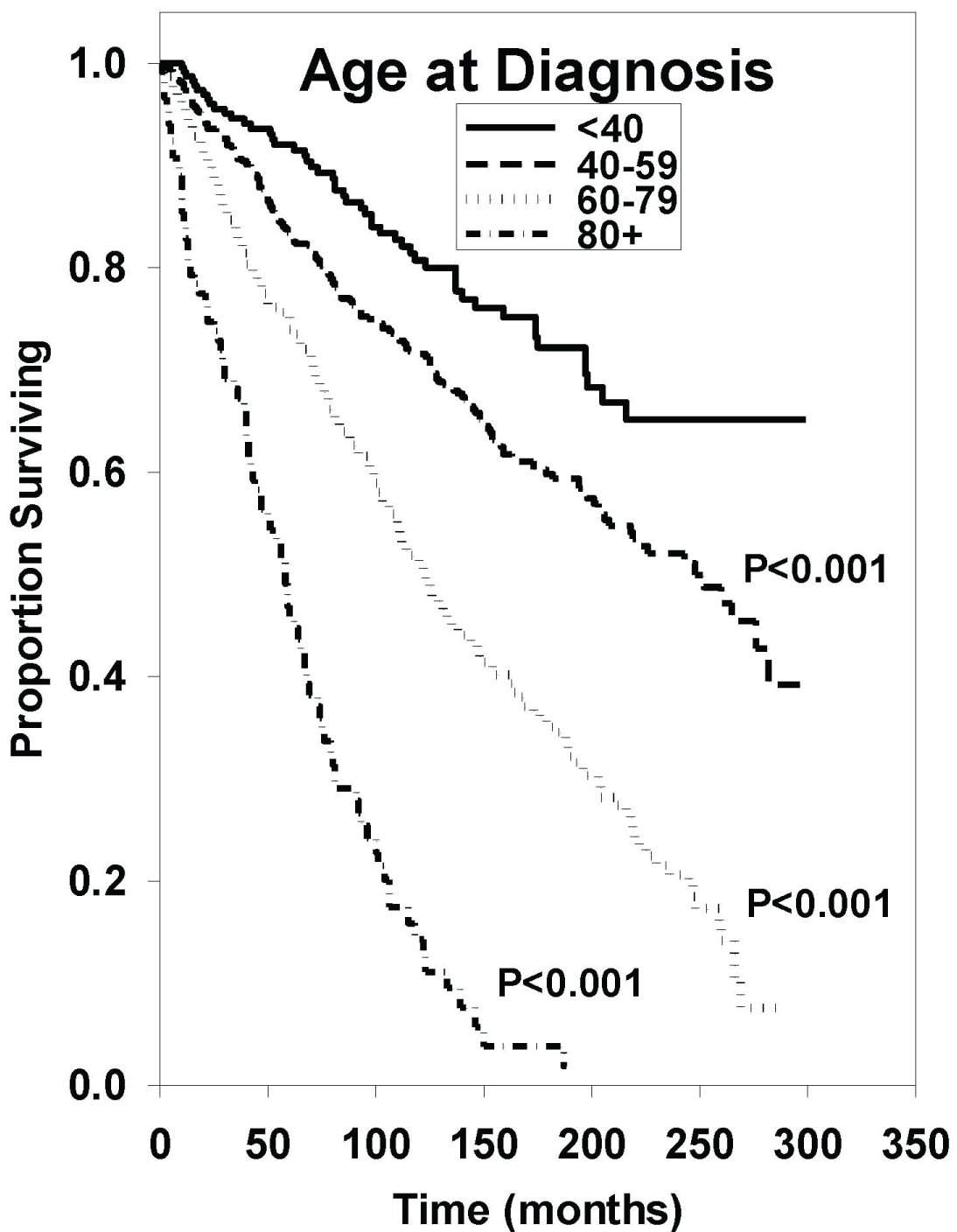 Figure 1: Survival curves based on univariate analysis of age groups. < 40 (n = 242), 40-59 (n = 687), 60-79 (n = 543), and 80+ (n = 123). P values were obtained using "age < 40" as reference group.
View Figure 1
Figure 1: Survival curves based on univariate analysis of age groups. < 40 (n = 242), 40-59 (n = 687), 60-79 (n = 543), and 80+ (n = 123). P values were obtained using "age < 40" as reference group.
View Figure 1
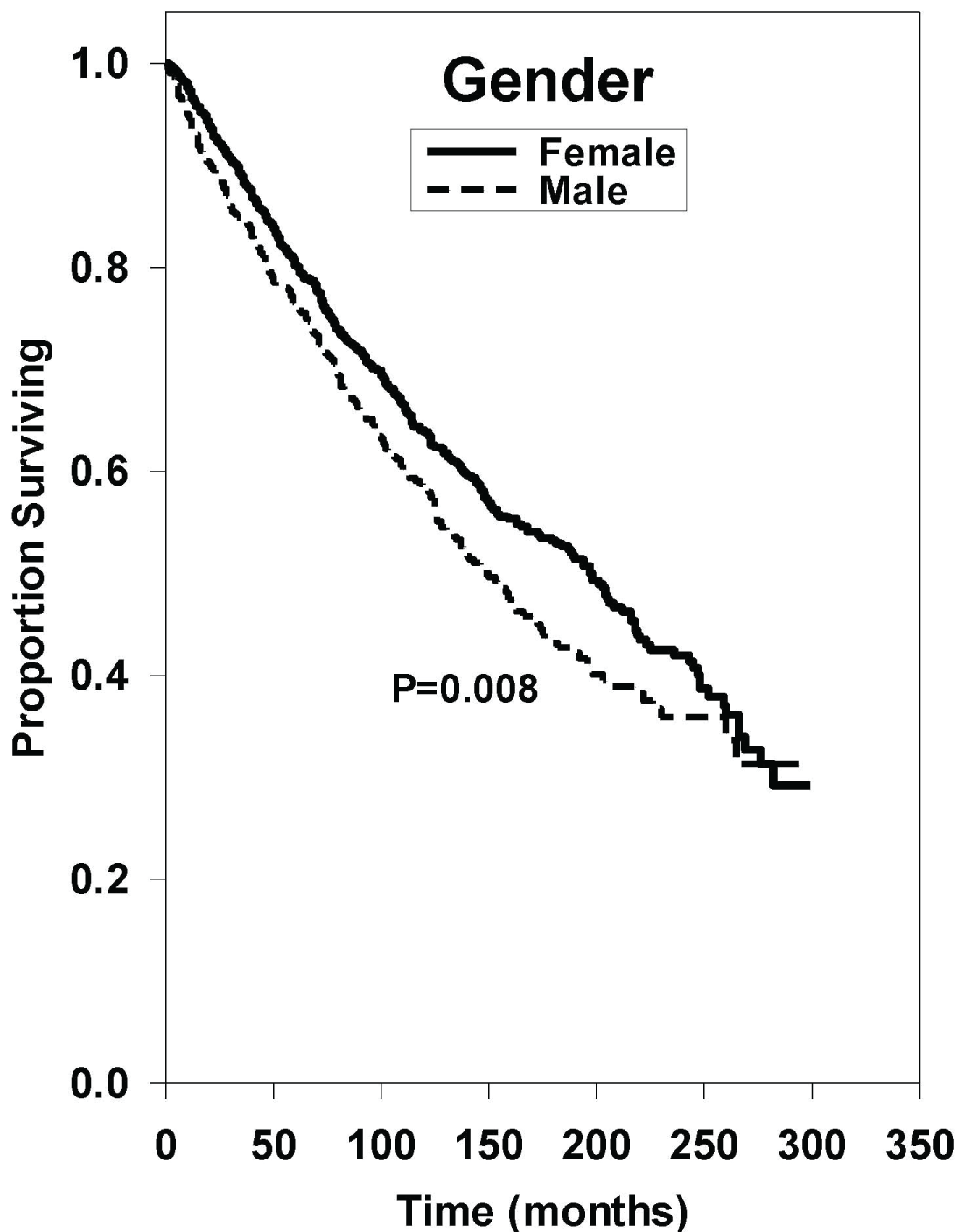 Figure 2: Survival curves based on univariate analysis of gender. Female (n = 952) and Male (n = 643). P value were obtained using "female gender" as reference group.
View Figure 2
Figure 2: Survival curves based on univariate analysis of gender. Female (n = 952) and Male (n = 643). P value were obtained using "female gender" as reference group.
View Figure 2
Table 2: Univariate median survival estimates (months) and hazard ratios. View Table 2
Median OS in patients who received radiation was 173 months, while patients who did not receive radiation had a median OS of 188 months as shown in Figure 3. The hazard ratio comparing these two groups yielded no statistically significant difference (HR: 0.95 [95% CI, 0.79-1.13], P = 0.571).
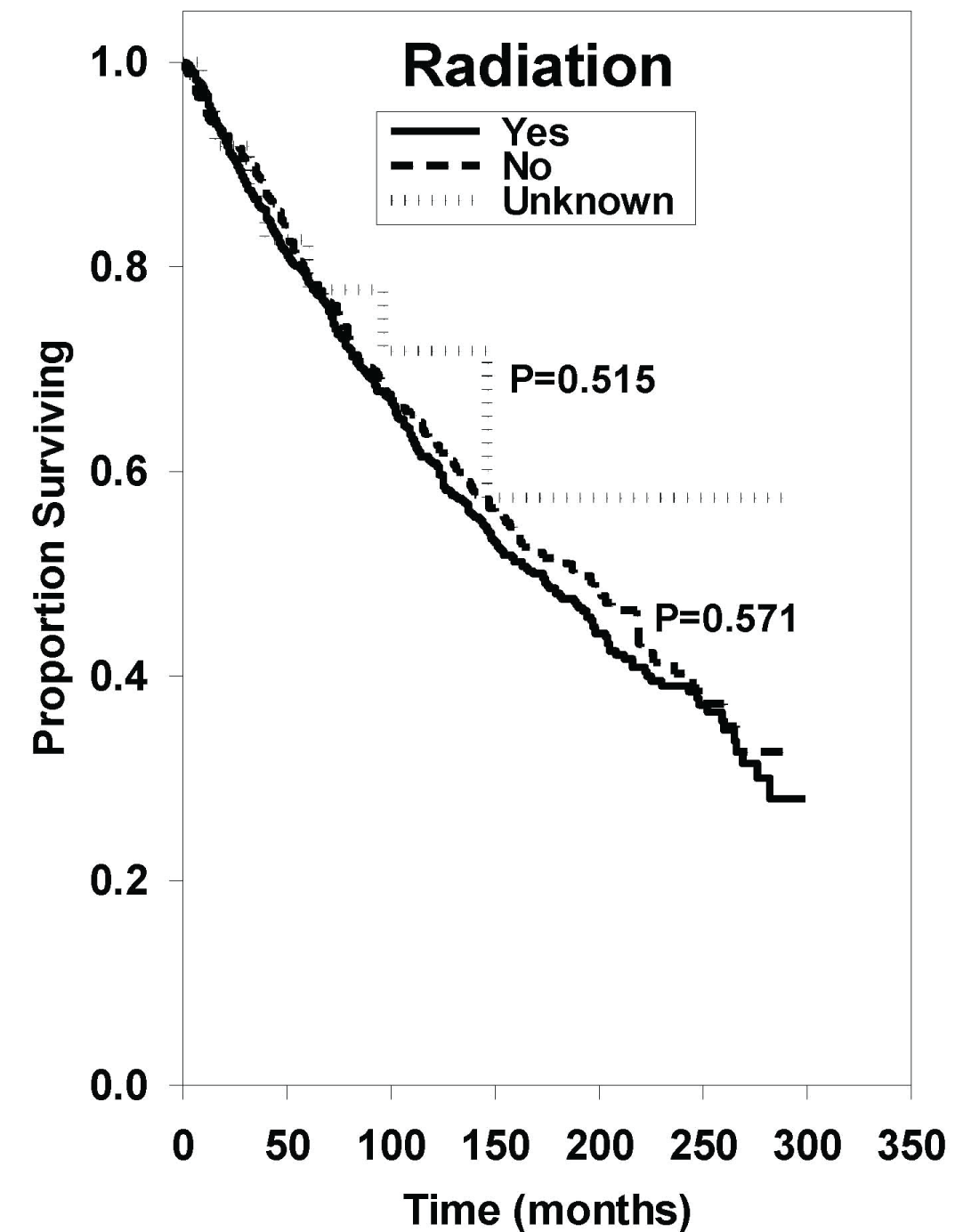 Figure 3: Survival curves based on univariate analysis of radiation treatment. Yes radiation (n = 1116), no radiation (n = 454), and unknown radiation (n = 25). P values were obtained using "yes radiation" as reference group.
View Figure 3
Figure 3: Survival curves based on univariate analysis of radiation treatment. Yes radiation (n = 1116), no radiation (n = 454), and unknown radiation (n = 25). P values were obtained using "yes radiation" as reference group.
View Figure 3
Of the patients who received surgery, median OS was 194 months while patients who did not receive surgery had a median OS of 71 months shown in Figure 4. The hazard ratio in patients who did not receive surgery was statistically worse as compared to patients who received surgery (HR: 2.72 [95% CI, 2.00-3.65], P < 0.001). Median OS between patients who received gross surgery and patients who received partial surgery was not statistically significant (HR: 0.92 [95% CI, 0.71-1.2], P = 0.566).
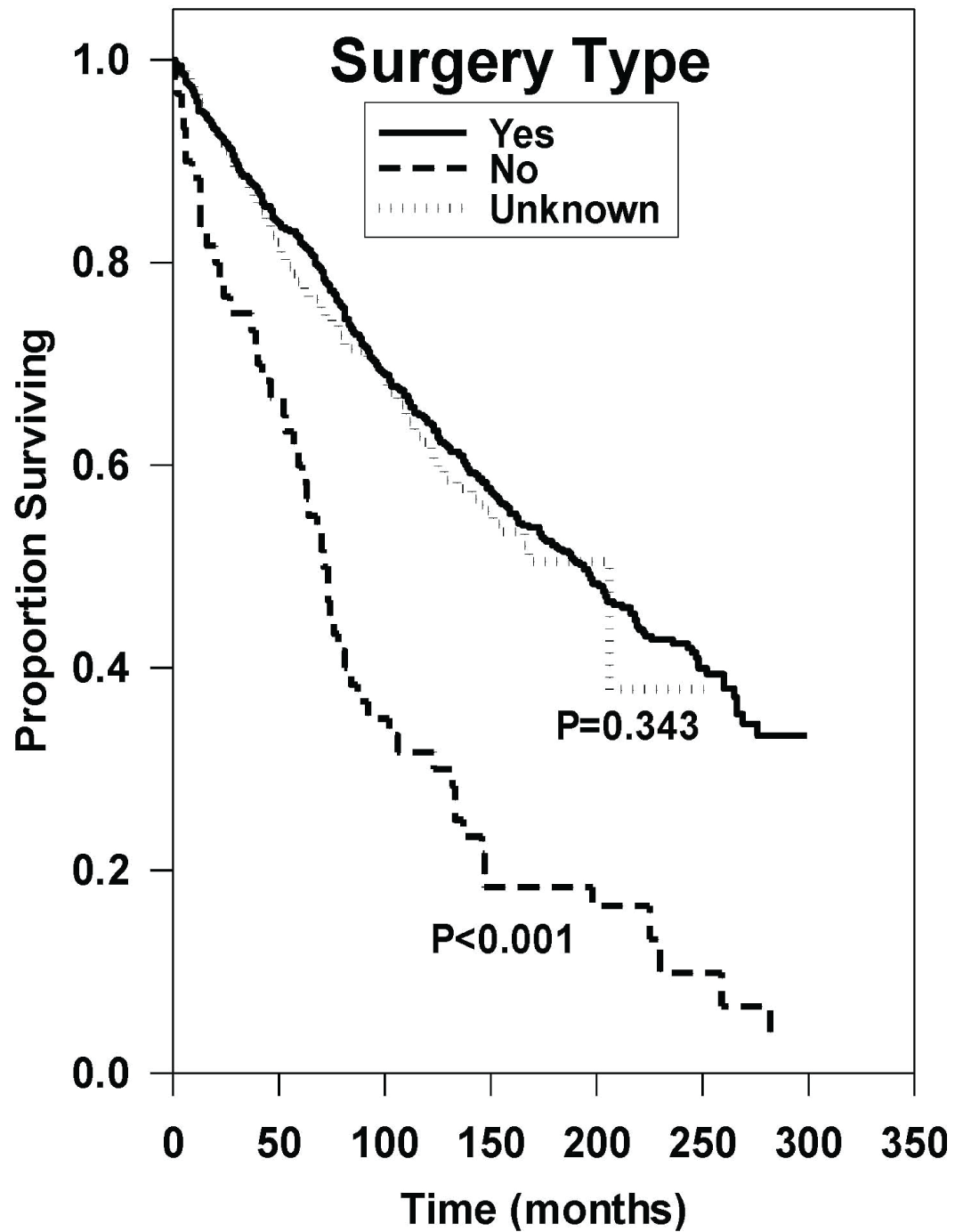 Figure 4: Survival curves based on univariate analysis of surgery type. Gross (n = 383), partial (n = 148), none (n = 62), and other/unknown (n = 1002). P values were obtained using "gross" as reference group.
View Figure 4
Figure 4: Survival curves based on univariate analysis of surgery type. Gross (n = 383), partial (n = 148), none (n = 62), and other/unknown (n = 1002). P values were obtained using "gross" as reference group.
View Figure 4
Patients with lymph node involvement had a median OS of 111 months, while patients without lymph node involvement had a median OS of 203 months. Figure 5 demonstrates a better prognosis for those without lymph node involvement (HR: 0.56 [95% CI, 0.48-0.66], P < 0.001).
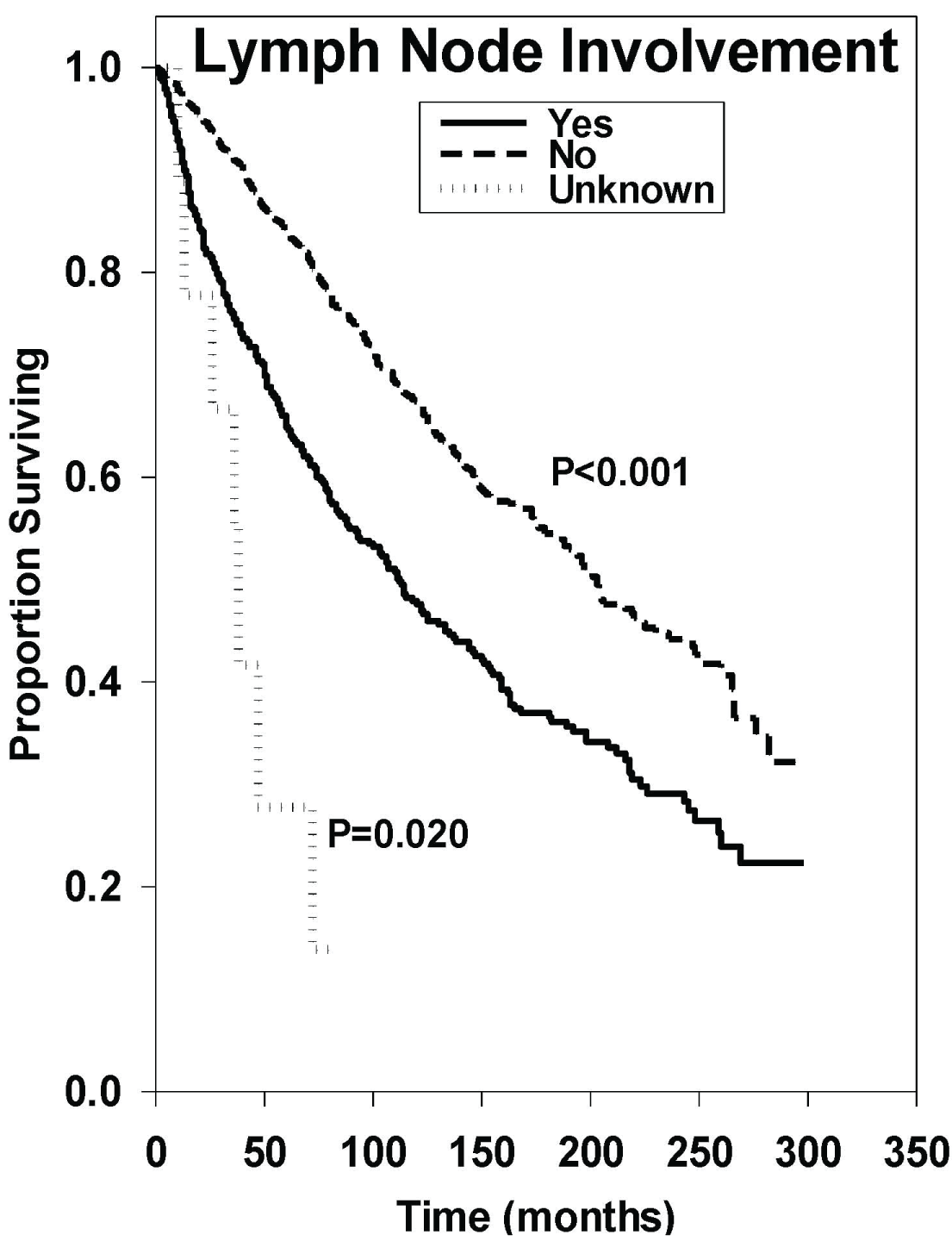 Figure 5: Survival curves based on univariate analysis of lymph node involvement. Yes (n = 392), no (n = 1194), and unknown (n = 9). P values were obtained using "yes" as reference group.
View Figure 5
Figure 5: Survival curves based on univariate analysis of lymph node involvement. Yes (n = 392), no (n = 1194), and unknown (n = 9). P values were obtained using "yes" as reference group.
View Figure 5
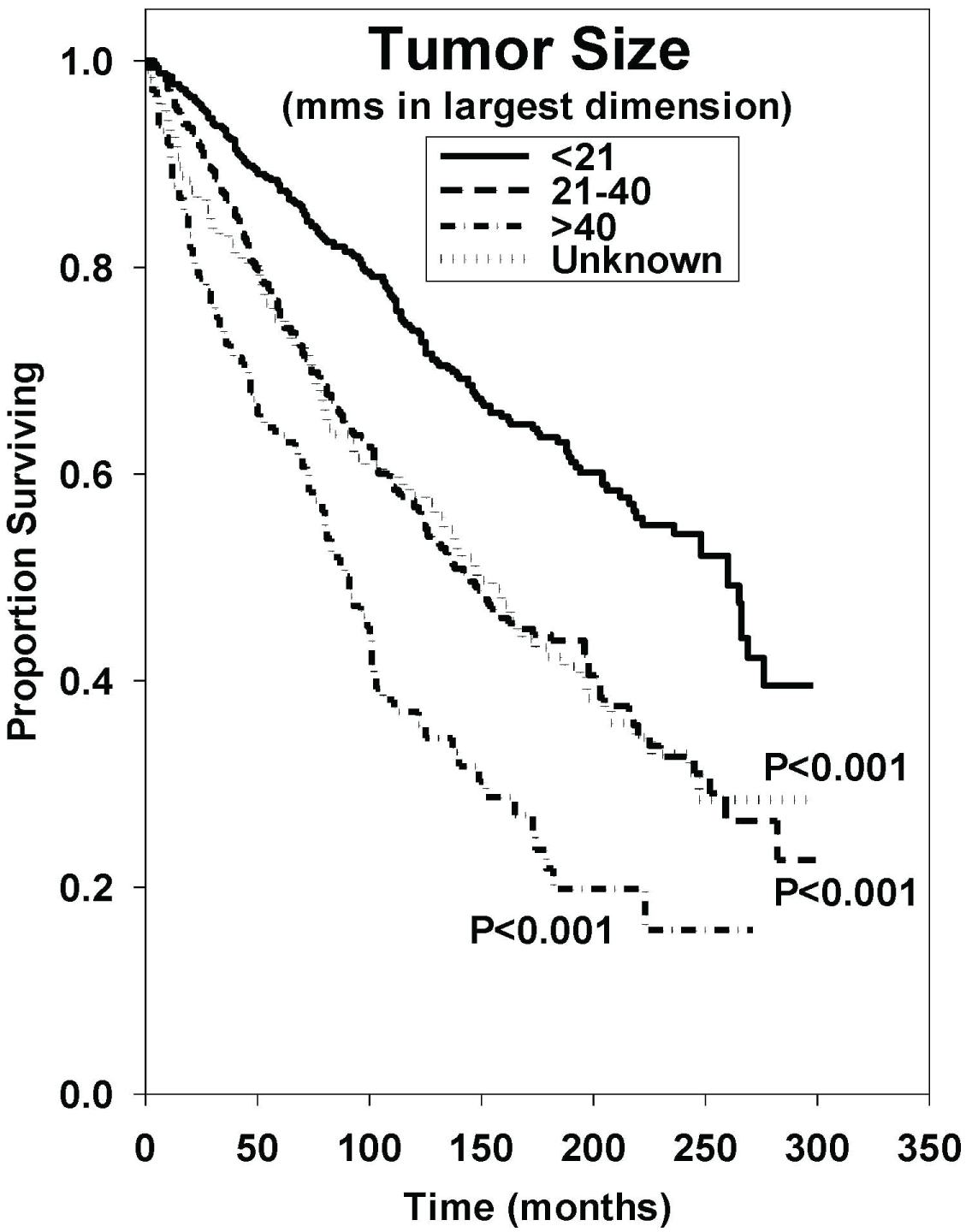 Figure 6: Survival curves based on univariate analysis of tumor size (mms in largest dimension). < 21 (n = 599), 21-40 (n = 586), > 40 (n = 181), and unknown (n = 229). P values were obtained using "< 21" as reference group.
View Figure 6
Figure 6: Survival curves based on univariate analysis of tumor size (mms in largest dimension). < 21 (n = 599), 21-40 (n = 586), > 40 (n = 181), and unknown (n = 229). P values were obtained using "< 21" as reference group.
View Figure 6
Both tumor size and site showed statistically significant effects on median OS. The effect of tumor size on median OS is shown in Figure 6. Patients with tumors that measured less than 21 mm at their largest dimension had a median OS of 260 months. Patients with tumors which measured between 21-40 millimeters in size had a median OS of 143 months, and patients with tumors which measured over 40 millimeters in size had a median OS 89 months. Using < 21-millimeter tumor size as the reference group, hazard ratios indicated inferior outcomes as tumor size increased (P < 0.001 in all cases). Of the tumor sites, tumors of the tongue and nasopharynx were associated with poorer outcomes (P < 0.001 in both cases).
Multivariate analysis of each subgroup is shown in Table 3. Similar to the univariate analysis of age at diagnosis, multivariate analysis showed progressively worsening outcomes as age at diagnosis increased (P < 0.001 in all cases). Regarding sex, males had a worse OS compared to females (HR: 1.41 [95% CI, 1.20-1.66], P = < 0.001). With regards to race, multivariate analysis revealed worse outcomes in those of other/unknown race (P = 0.009).
Table 3: Multivariate hazard ratios, confidence intervals, and P values. View Table 3
Analysis of those who received RT and those who did not revealed no statistical significant difference in OS between groups. However, multivariate analysis did reveal statistically significant results between individuals who received gross surgical resection and partial surgical resection, with a superior outcome in those who received partial resection (HR: 0.91 [95% CI, 0.85-0.98], P = 0.01).
Similar to the univariate analysis, multivariate analysis revealed a superior outcome in patients without lymph node involvement compared to those with lymph node involvement (HR: 0.62 [95% CI, 0.52-0.73], P < 0.001). In addition, it also indicated a poorer prognosis as tumor size increased (P < 0.001 in all cases).
Using major salivary glands as a reference group, multivariate analysis revealed a superior outcome in those with a tumor located in the palate (HR: 0.99 [95% CI, 0.97-1.00], P = 0.04). Inferior outcomes were indicated with tumor sites located in the unspecified mouth part, tongue, and nasopharynx (P < 0.001 in all cases).
Overall survival rates are shown in Table 4. In our cohort, we found that in 5 years there was a 78.6 percent survival rate (95% CI, 76.3-80.6).
Table 4: Absolute survival rates at 0.5, 1, 2, 5, 10, 15 and 20 years. View Table 4
A standard treatment protocol for patients with ACC of the head and neck based on level I evidence has yet to be established due to a lack of research regarding treatment modalities and OS in patients with ACC. Our study attempts to elucidate the effects of patient and treatment variables on OS. Our data shows significant differences in OS in the following variables: age at diagnosis, gender, whether or not the patient received surgery, type of surgery received, lymph node involvement, tumor size, and tumor site.
A recent SEER database study of 1,435 patients by Shen, et al. calculated cumulative incidence function for cause of death in patients with head and neck ACC, which similarly demonstrated that age, primary tumor size, lymph node involvement, distant metastasis, and surgery are all predictors of both cause-specific death and death from other causes [7]. Regarding age, Shen, et al. found that cumulative incidence of 5-year death increased as age of diagnosis increased [7]. This is consistent with our data, which shows that OS worsens as age at diagnosis increased. Additionally, Ko, et al. found that patients greater than 60 years of age at the time of diagnosis had worse survival outcomes compared to those less than 60 years of age in a single-site study of 60 patients with head and neck ACC [8]. Our study also revealed a decrease in OS in males (HR:1.41 [95% CI, 1.20-1.66], P < 0.001). This is consistent with the findings of a SEER study of 3,026 patients with head and neck ACC by Ellington, et al. which also revealed that men have a worse OS [9].
Statistical analysis showed that RT failed to improve OS in our patient population. Data surrounding the use of radiation as a treatment modality for patients with ACC of the head and neck has had mixed results. Consistent with our finding, the SEER study by Ellington, et al. also showed that RT provided no benefit in OS [9]. Although these results suggest that RT does not improve OS, RT may have clinical benefit regarding local control. Ali, et al. found that in a retrospective cohort study of 87 patients, those with head and neck ACC that received adjuvant RT were less likely to have local recurrence [10]. In a study of 35 patients with salivary gland tumors, including ACC subtype, RT similarly showed improved local control [11].
Surgical resection was found to be associated with improved OS when compared to those that were not treated with surgery. Furthermore, our study showed that patients who received partial resection had better outcomes than those that received gross surgical resection. In a review of current literature, surgery appears to be universally associated with better outcomes for patients with head and neck ACC. The two other large SEER studies on ACC by Shen, et al. and Ellingson, et al. both showed similar results [7,9]. Additional research regarding surgery type (gross resection vs. partial resection) and clinical outcomes is a possible avenue for future study.
Lymph node involvement was found in 392 patients in our study population and was notable for a decrease in OS. Findings of lymph node involvement in other studies also showed a decrease in OS. In an institutional study of 66 patients, Gandhi, et al. found that head and neck ACC patients with lymph node involvement was associated with a poor disease-free survival (HR: 4.06 [95% CI, 1.63-26.17], P = 0.006) [12]. A retrospective study of 54 patients with ACC of the tongue by Han, et al. also found that lymph node involvement was a poor prognosticator [13]. Additionally, the SEER study by Shen, et al. showed progressively higher cumulative incidences of death as N stage increased [7].
Our study showed a progressive decrease in OS as tumor size increased. These findings are consistent with the conclusions of the SEER study by Shen, et al. [7]. In addition, a retrospective study of 565 patients by Terhaard, et al. revealed that patients with a primary tumor size which measured greater than six centimeters had a decrease in OS compared to those with a tumor size which measured less than two centimeters [14]. These findings were also supported by SEER database studies by Lee, et al. and Lloyd, et al., both of which demonstrated a decrease in OS as tumor size increased [15,16].
We found that primary tumors of the major salivary glands and palate are associated with improved OS, likely due to early detection of palpable abnormal growth and ease of surgical approach. A 45-case series by Lukšić, et al. also demonstrated better OS in patients with ACC of major salivary glands compared to other sites [17]. In addition, the SEER study by Ellington, et al. found that "primary tumor location in the neck/pharynx when compared with a primary site in the salivary glands (HR: 1.62, [95% CI, 1.25-2.01]) were significant predictors of decreased survival" [9]. However, the SEER study by Shen, et al. failed to show differences in cumulative incidence of death between the major and minor salivary gland sites [7].
ACC of the head and neck is an uncommon histological subtype salivary gland cancer that is commonly seen in community tertiary care centers. Due to the rarity of this subtype and lack of clinical data, a standardized treatment protocol has not yet been established. There is a paucity of level I evidence based on phase III trials. Information gained from large population database can be very informative for clinicians making treatment decisions. Our study revealed that younger age at diagnosis, female sex, absence of lymph node involvement, small tumor size, major salivary gland and palate tumor site, and surgical resection were all positive predictors of improved OS.
Previous research has shown RT to have a local control advantage in some patient groups. However, our study did not show that RT had a significant effect on OS. The use of multivariate analysis in our study attempted to control for any differences between patients who received RT and those that did not. In general, RT in ACC is usually reserved for patients with more advanced disease. It is possible that our analysis was unable to completely control for clinical differences in patients that received RT and those that did not. Our study is limited due to its retrospective nature, and further research is required to determine whether specific subgroups of patients with advanced disease may, in fact, benefit from RT with respect to OS.
In addition, more studies are needed to clarify potential benefits of partial vs. gross surgery type, combined treatment modalities, and the effects of these treatments on local control and distant metastasis.
This work was funded in part by the Community Cancer Fund, Spokane, WA
No authors of this paper have any financial disclosures to make.
All authors listed on this contributed to the design and implementation of the research, to the analysis of results, and to the writing of this manuscript.