Background: Pancreatic ductal adenocarcinoma (PDAC) remains one of the most aggressive and challenging cancers, causing substantial mortality worldwide. Despite advancements in treatment modalities, the prognosis for patients with PDAC remains dismal, emphasizing the critical need for identifying factors influencing early recurrence and survival post-surgery. This study aimed to clarify the risk factors for early recurrence (within 1 year) in patients who underwent upfront surgery for resectable PDAC.
Methods: We retrospectively enrolled 127 patients who underwent upfront R0/R1 resection for resectable PDAC at our institute between January 2005 and December 2019. We collected clinicopathological background data and performed univariate and multivariate analyses to evaluate the risk factors for early recurrence.
Results: The median follow-up period was 27 months. The overall and recurrence-free survival rates at 1, 3, and 5 years were 82%, 56%, and 33% and 68%, 33%, and 24%, respectively. Multivariate analysis revealed that CA19-9 ≥ 50U/mL and positive anterior peripancreatic surface invasion (T3) were independent risk factors. A prognostic staging model using one point for each risk factor provided a well-categorized predictive model. When all patients were classified into three groups (Score 0: neither CA19-9 ≥ 50 nor T3 factors; Score 1: one factor; and Score 2: both factors), the 5-year overall survival rates were 45%, 41%, and 22% for scores of 0, 1, and 2, respectively (p < 0.001). The corresponding 5-year recurrence-free survival rates were 45%, 30%, and 9%, respectively (p < 0.001).
Conclusion: High preoperative CA19-9 level and peripancreatic infiltration were independent risk factors for early recurrence after upfront surgery.
Early recurrence, Neoadjuvant chemotherapy, Pancreatic ductal adenocarcinoma, Upfront surgery
PDAC: Pancreatic Ductal Adenocarcinoma; NAC: Neoadjuvant Chemotherapy; BR-PDAC: Borderline Resectable Pancreatic Ductal Adenocarcinoma; NCCN: National Comprehensive Cancer Network; R-PDAC: Resectable Pancreatic Ductal Adenocarcinoma; UICC: Union International Contre le Cancer staging system; PV: Portal Vein; SMA: Superior Mesenteric Artery; CA: Celiac Artery; CHA: Common Hepatic Artery; CEA: Carcinoembryonic Antigen; CA19-9: Carbohydrate Antigen; N: lymph node metastasis; CH: Common Bile Duct Invasion; DU: Duodenal Invasion; S: anterior peripancreatic surface invasion; RP: Retroperipancreatic Margin; PV: Portal Vein Invasion; A: Arterial System Invasion; PL: Extra-Pancreatic Nerve Plexus Invasion; ly: lymphoid infiltration; v: venous infiltration; ne: nerve infiltration; OR: Odds Ratio; 95% CI: 95% Confidence Interval; ROC: Receiver Operating Characteristic; AUC: Area under the Receiver Operating Characteristic Curve; OS: Overall Survival; RFS: Recurrence-Free Survival; PD: Pancreaticoduodenectomy; DP: Distal Pancreatectomy; TP: Total Pancreatectomy; GEM: Gemcitabine; S-1: combined tegafur, gimestat, and otastat potassium; GS: GEM+S-1; nabPTX: nab-paclitaxel
Pancreatic ductal adenocarcinoma (PDAC) is currently the seventh leading cause of cancer-related deaths worldwide and the fourth most common cause of cancer-related deaths in Japan [1]. In addition, the number of PDACs is increasing yearly [2]. PDAC is an aggressive type of cancer, with a poor prognosis and an overall 5-year survival rate of approximately 10% [3]. Despite improved efforts to achieve curative surgery, recurrence after pancreatic resection remains frequent and is observed within the first 6 months in approximately 20% of patients [3].
Adjuvant chemotherapy after surgical resection can prolong survival versus surgical resection alone. Recently, neoadjuvant chemotherapy (NAC) has gained attention for improving prognosis more than adjuvant chemotherapy alone in surgery-eligible patients with PDAC [3,4]. NAC is recommended for borderline resectable PDAC (BR-PDAC), as diagnosed by the clinical practice guidelines for pancreatic cancer 2019 and the National Comprehensive Cancer Network (NCCN) guidelines [3-5].
For patients with resectable PDAC (R-PDAC), NAC is recommended when high-risk features are present, [4-6] while upfront surgery is indicated for patients without high-risk features [7]. Although various reports on high-risk features in patients with R-PDAC have been published, the high-risk features for NAC in R-PDAC remain controversial [8-10]. The risk factors for early recurrence in patients undergoing upfront surgery for R-PDAC remain unclear.
This study aimed to clarify the long-term outcomes after upfront surgery in a large cohort of patients with R-PDAC with long-term follow-up. We also aimed to identify the risk factors for early recurrence (within 1 year) in patients undergoing upfront surgery for R-PDAC.
A total of 131 consecutive patients with R-PDAC underwent pancreatic resection at our department between January 2005 and December 2019. Among these patients, two died during the postoperative hospital stay, and two were lost to follow-up within 6 months after surgery. We excluded these four patients from the study and enrolled the remaining 127 patients. We retrospectively analyzed the characteristics, clinicopathologic features, and long-term outcomes of the 127 patients with R-PDAC. All patients underwent curative pancreatic resection with resection status of R0 (no residual tumor) or R1 (with microscopic residual tumor). R-PDAC was defined according to the 7 th edition of the Union International Contre le Cancer staging system (UICC) as a tumor that is not in contact with the superior mesenteric vein or portal vein (PV), or the contact/infiltration is less than 180° and no obstruction is observed. Clear adipose tissue was observed between the superior mesenteric artery (SMA), celiac artery (CA), common hepatic artery (CHA), and the tumor, and no contact or infiltration [11]. Preoperative evaluation included determining the age, sex, presence of diabetes mellitus, drinking habits, and two tumor markers, namely, carcinoembryonic antigen (CEA) and carbohydrate antigen 19-9 (CA19-9). The serum concentrations of CEA and CA19-9 were measured within 1 week before surgery. We generally determined the surgical procedure based on tumor localization using preoperative computed tomography images and endoscopic ultrasound. Early recurrence was defined as recurrence within 1 year. We compared the early recurrence group with others and analyzed the risk factors. Recurrence patterns were classified into three types: Distant metastasis (liver, lung, extra-regional lymph node, peritoneal dissemination, or No.16 lymph node), local recurrence (No.14 lymph node, SMA plexus, CHA plexus, or remnant pancreas); and both (distant metastasis and local recurrence). This study was approved by our institution’s Ethics Committee (approval number-20C153), and all patients provided informed consent.
Postoperative pathological diagnosis was defined according to the seventh edition of the UICC. Based on general rules, we defined tumor size, lymph node metastasis (N), common bile duct invasion (CH), duodenal invasion (DU), anterior peripancreatic surface invasion (S), retroperipancreatic margin (RP), portal vein invasion (PV), arterial system invasion (A), extra-pancreatic nerve plexus invasion (PL), lymphoid infiltration (ly), venous infiltration (v), nerve infiltration (ne), histological differentiation type, and pathological stage.
Chi-squared and Fisher’s exact tests were used to compare nonparametric categorical variables. The Mann-Whitney U test was used to compare nonparametric continuous variables. A logistic regression model was used for multivariate analyses of factors related to early recurrence and odds ratio (OR) and 95% confidence interval (95% CI) calculation. The cut-off values of the predictive scores of CEA, CA19-9, and tumor diameter were calculated using a receiver operating characteristic (ROC) curve. The ROC curve is a plot of sensitivity values 1-specificity for all possible cut-off values. The bootstrap bias-corrected area under the ROC curve (AUC) was used as a measure of the predictive performance of each risk factor. The serum CEA and CA19-9 levels and tumor size were used to plot the ROC curve for early recurrence. The AUCs of serum CEA level, serum CA19-9 level, and tumor size were 0.630, 0.649, and 0.662, respectively. The best cut-off values calculated from the ROC analysis were 2.9 ng/ml, 50 U/ml, and 25 mm for serum CEA, CA19-9 level, and tumor size, respectively. Overall survival (OS) was defined as the interval between the date of surgery and death or the last follow-up if death did not occur. Recurrence-free survival (RFS) was defined as the interval from surgery to the date of diagnosis of the first recurrence or last follow-up. OS and RFS curves were calculated using the Kaplan-Meier method and compared using the log-rank test. We used a Cox proportional hazards model for univariate and multivariate analyses of the prognostic factors related to OS and RFS. All P values were derived from two-tailed tests, and P < 0.05 was considered statistically significant. All statistical analyses were performed using the JMP 16 software package (SAS Institute Inc., Cary, NC, USA).
Table 1 shows the clinicopathological characteristics of the patients enrolled in this study. The 127 patients included 61 men (48%) with a median age of 73 years (range, 48-89 years). The preoperative median CEA was 3.5 ng/ml (range, 0.5-39), and CA19-9 was 53 U/ml (range, 1-11153). Pancreaticoduodenectomy (PD) was performed in 64, distal pancreatectomy (DP) in 60, and total pancreatectomy (TP) in three patients. The median maximum tumor size was 24 mm (range 4-110). Final staging comprised tumors of stage IA (n = 18), IB (n = 5), IIA (n = 43), IIB (n = 58), III (n = 1), and IV (n = 1). Adjuvant chemotherapy was administered to 72 patients (57%). Chemotherapeutic agents were gemcitabine (GEM) in 25 cases; combined tegafur, gimestat, and otastat potassium (S-1) in 45 cases; GEM+S-1 (GS) in one case; and nab-paclitaxel (nabPTX) + GEM in one case.
Table 1: Clinical characteristics of the patients. View Table 1
The median follow-up duration was 26 months (range, 3-157 months). The OS rates for all patients were 82%, 56%, and 33% at 1, 3, and 5 years, respectively (Figure 1a). The 1-, 3-, and 5-year RFS rates were 68%, 33%, and 24%, respectively (Figure 1b). During the observation period, recurrence was observed in 75 patients (59%). Among these, 33 patients developed recurrence within 1 year of surgery and were enrolled in the early recurrence group (Table 2). In the early recurrence group, CA19-9 level was significantly higher (p = 0.021), with high proportions of stage III tumors (p = 0.009), large diameters (p = 0.002), and extra-pancreatic infiltration with lymph node metastasis (p = 0.005). The pattern of metastasis at the recurrence site did not vary significantly between the two groups (p = 0.175).
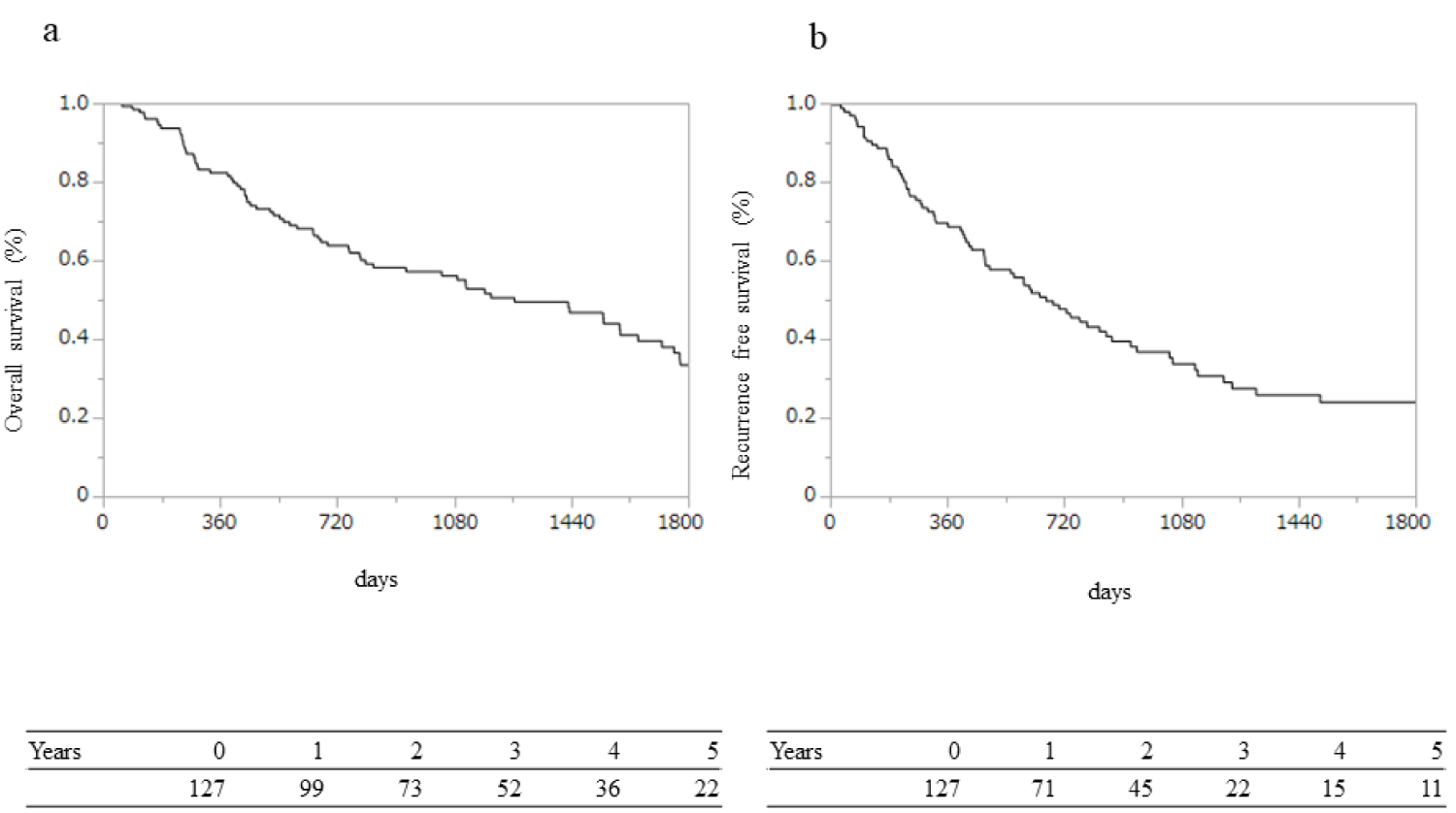 Figure 1: Long-term survival and recurrence outcomes: (a) Overall survival (OS) and (b) Recurrence-free survival (RFS). OS rates were 82%, 56%, and 33%, and RFS rates were 68%, 33%, and 24% at 1, 3, and 5 years, respectively.
View Figure 1
Figure 1: Long-term survival and recurrence outcomes: (a) Overall survival (OS) and (b) Recurrence-free survival (RFS). OS rates were 82%, 56%, and 33%, and RFS rates were 68%, 33%, and 24% at 1, 3, and 5 years, respectively.
View Figure 1
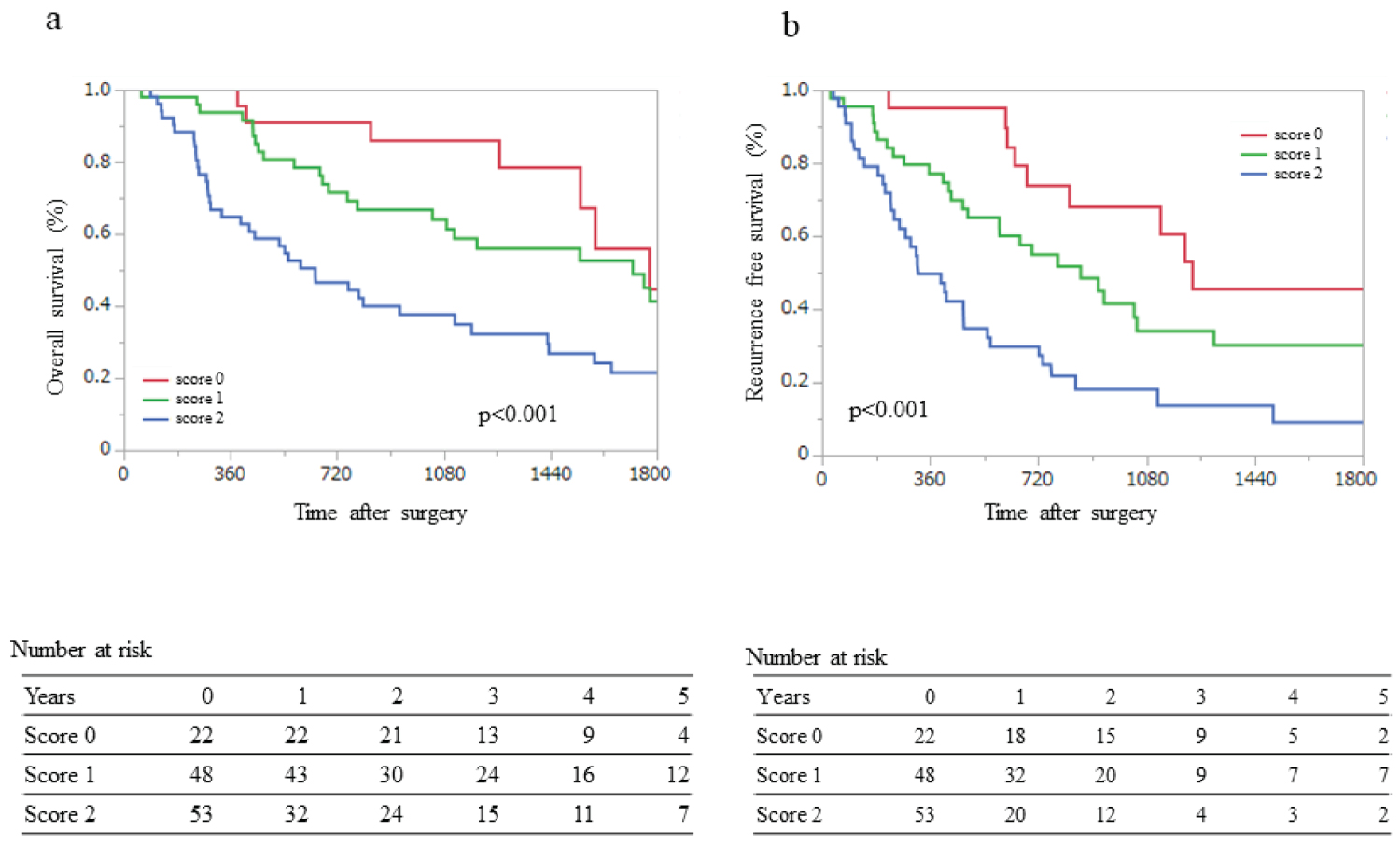 Figure 2: Overall survival (OS) and recurrence-free survival (RFS) for the three groups.
Figure 2: Overall survival (OS) and recurrence-free survival (RFS) for the three groups.
Score 0: neither CA19-9 ≥ 50 nor T3; Score 1: CA19-9 ≥ 50 or T3; Score 2: CA19-9 ≥ 50 and T3 (a) The OS rates at 1, 3, and 5 years were 95%, 86%, and 45% for Score 0; 92%, 61%, and 41% for Score 1; and 63%, 38%, and 22% for Score 2, respectively; (b) The RFS rates at 1, 3, and 5 years were 90%, 60%, and 45% for Score 0; 75%, 30%, and 26% for Score 1; and 47%, 14%, and 9% for Score 2, respectively.
View Figure 2
Table 2: Patient backgrounds: early recurrence versus other groups. View Table 2
Table 3 shows the clinicopathological characteristics and findings of the univariate analysis for early recurrence. Multivariate analysis identified serum CA19-9 level ≥ 50 U/ml (OR: 3.97; 95% CI: 1.40-11.24; p = 0.001) and S positivity (OR: 4.95; 95% CI: 1.51-16.3; p = 0.009) as independent factors for early recurrence (Table 3). OS and RFS were also stratified using CA19-9 ≥ 50, and S-positive status (T3) was extracted using multivariate analysis. A prognostic staging model using four risk factors for OS and RFS, with or without serum levels of CA19-9 ≥ 50 U/ml and with or without T3, was scored as 0 or 1, respectively, yielding a total score of 0 to 2. We classified the patients into three groups, namely, Score 0, neither CA19-9 ≥ 50 nor T3; Score 1, CA19-9 ≥ 50 or T3; and Score 2, CA19-9 ≥ 50 and T3. OS and RFS analysis revealed that lower scores in this prognostic model were associated with better survival and fewer recurrences after surgery (Figure 2a and Figure 2b). The 1-, 3-, and 5-year OS rates were 100%, 86%, and 79% for Score 0; 90%, 64%, and 54% for Score 1; and 66%, 40%, and 28% for Score 2, respectively (p < 0.001; Figure 2a). The 1-, 3-, and 5-year RFS rates were 95%, 68%, and 45% for Score 0; 79%, 48%, and 30% for Score 1; and 57%, 18%, and 9% for Score 2, respectively (p < 0.001; Figure 2b).
Table 3: Univariate and multivariate analysis for early recurrence. View Table 3
This study examined the risk factors for early recurrence after upfront surgery for R-PDAC . We found that high CA19-9 levels and pathological peripancreatic infiltration were risk factors. Moreover, we discovered that patients with high CA19-9 levels and/or pathological peripancreatic infiltration had poor survival, and we stratified OS and RFS after upfront surgery for R-PDAC.
Current treatment strategies based on a combination of surgery, chemotherapy, and radiotherapy have yielded an unsatisfactory 5-year survival rate of approximately 10% for patients with PDAC [1]. In addition, the 5-year survival rate was reported as 15-40% in patients with PDAC who underwent surgical resection, although s urgical resection offers the only chance for cure [12].
Because of the unsatisfactory survival benefit provided by surgical resection alone, multiple treatment strategies for localized PDAC are recommended [13]. According to the clinical practice guidelines for pancreatic cancer 2019 and NCCN, NAC is recommended for BR-PDAC [4,5,14]. In patients with R-PDAC, NAC is recommended for those with high-risk features. Therefore, upfront surgery is indicated only for patients without high-risk features. Several reports have discussed the high-risk features of R-PDAC. However, these features remain controversial because the risk factors for early recurrence in patients undergoing upfront surgery remain unclear. Therefore, we investigated these risk factors for early recurrence and survival in patients who underwent upfront surgery for R-PDAC.
Two tumor markers for PDAC, CEA and CA19-9, are used individually for detecting tumors and are useful for evaluating tumor invasiveness or prognosis [15,16]. This study used multivariate analysis to demonstrate that a high CA19-9 level was a significant risk factor for early recurrence. Several studies have correlated CA19-9 serum levels with PDAC progression, which aligns with our results [16-19]. Previous reports have set various cut-off values as median values, values calculated from ROC analysis, upper or lower normal limit values, and values based on other reports. In this study, we defined a cut-off value of 50 ng/ml for CA19-9 as calculated from ROC analysis, and CA19-9 ≥ 50 U/ml was identified as an independent risk factor for early recurrence.
Multivariate analysis also revealed that the serosal aspect of anterior pancreatic tissue invasion, (S)-positive (T3), was a significant risk factor for early recurrence. According to the 7 th edition of the General Rules for the Study of Pancreatic Cancer developed by the Japan Pancreatic Society, T3 indicates that tumor infiltration has progressed beyond the pancreas but not to the celiac or superior mesenteric arteries [20]. Locoregional tumor extension correlates with recurrence and survival in patients with PDAC. Accordingly, preoperative imaging evaluation of tumor extension is important when selecting appropriate treatment strategies. As the diagnostic imaging system has improved in recent years, this can be easily evaluated using preoperative computed tomography images and endoscopic ultrasound [16-19,21].
Notably, this study proposed a prognostic model using two risk factors (CA19-9 and peripancreatic infiltration) for early recurrence. To maximize clinical utility, this model includes only variables that are available before surgery. Using this model may enable accurate prediction of survival and recurrence after upfront surgery for R-PDAC. The RFS rate after upfront surgery was significantly higher in patients scoring 0 than in those scoring 1 or 2. Thus, the proposed model was a valuable prognostic predictor in patients with R-PDAC who underwent upfront surgery, according to our results. This model could be of important clinical value for patients with R-PDAC who are to undergo upfront surgery.
In the absence of clear guidelines, the biases that often determine treatment with NAC versus upfront surgery for R-PDAC include tumor size, degree of vascular invasion, patient and tumor conditions, preoperative imaging, and tumor markers, which are determined independently for each institution [15,22]. NAC may have advantages, including treatment of micrometastasis, downstaging, increased R0 resection rates, and better patient selection, owing to the exclusion of patients from biologically aggressive disease [4,23-25]. Therefore, NAC should be indicated in high-risk cases according to imaging findings, elevated CA19-9, large tumors, enlarged local lymph nodes, excessive weight loss, and extreme pain [8,26-28]. These results suggest that various features influence early recurrence and poor prognosis, and previous reports have attempted to predict early recurrence using various factors [15-19,21]. In this study, we used only two variables that were easy to judge using preoperative clinical laboratory tests and findings. Our simple prognostic model may help in selecting the appropriate multidisciplinary treatment for R-PDACs.
This study had several limitations. First, it was a retrospective cohort study of patients who underwent pancreatectomy; thus, selection bias may have occurred. Second, patient backgrounds could not be matched because the experiment was performed in a single institution. Third, postoperative chemotherapy regimens were combined in this study because the postoperative adjuvant chemotherapy regimen was changed from GEM to S-1 after the JASPAC01 trial [29-32]. Despite these limitations, we believe that our results are clinically informative and that similar investigations of therapeutic strategies for R-PDAC should continue. In conclusion, this study investigated the feasibility of stratifying R-PDAC patients based on poor prognostic factors. We identified high CA19-9 levels and pathological peripancreatic infiltration as significant risk factors for early recurrence after upfront surgery for R-PDAC. Using these two risk factors, we developed a simple prognostic model related to long-term survival after surgery. Patients with high CA19-9 levels and/or pathological peripancreatic infiltration had poor survival rates. This model could be useful for identifying groups with poor prognoses and selecting appropriate treatment strategies for patients with R-PDAC. Further clinical studies are required to confirm these results.
We would like to thank Editage (www.editage.jp) for English language editing.
The authors declare there are no conflicts of interest.