Background and aims: Measurement of actual concentration of IgG requires methods like heat inactivation (HI) of plasma. This study was aimed at comparing of heat treated ABO titers performed by conventional test tube technique (CTT) and column agglutination technique (CAT) with HA/SPRCA.
Materials and methods: This was a prospective, observational study conducted from October 2018 to March 2020. All consecutive O group donors who gave consent for participation were included. All samples were tested by CTT and CAT before and after HI (pCTT, pCAT) and with HA/SPRCA.
Results: A total of 2005 donors were included. IgG titers were found to be more than IgM titers. PCTT IgG results are similar or lower when compared to results obtained by SPRCA, while pCAT IgG titers were higher with pCAT when compared to HA/SPRCA. Results of titers obtained by pCTT were lower as compared to pCAT and HA/SPRCA, with majority giving results less than 64. Median IgG and IgM titers for both anti-A and anti-B were highest in pCAT, while median IgG and IgM anti-A and anti-B titers were similar with HA and pCTT.
Conclusion: Results obtained by HA/SPRCA were closer to results obtained by pCTT, with the advantage of less time consumption, automation requiring less expertise and no inter-observer variation. Titers obtained by pCAT were higher in comparison to HA/SPRCA and pCTT results, due to high sensitivity.
Conventional tube technique, Column agglutination technology, ABO, Titration, Heat inactivation
The two most important immunoglobulin types in transfusion medicine are IgG and IgM. Anti-A and anti-B of blood groups A and B are predominantly IgM type while those of blood group O are predominantly of IgG type. [1]. Determination and monitoring of ABO isohemagglutinin titers play an important role in the outcome of solid organ or hematopoietic stem cell transplant [1]. It is the IgG type antibodies which are clinically significant and play a pivotal role during solid organ or hematopoietic stem cell transplant. Their measurement is of extreme importance as their concentration regulates immune reactions related to transfusion and transplantation and hence, measurement of IgG antibodies alone becomes important [2-9].
The concentration of IgG antibodies can be masked by IgM antibodies which can lead to over estimation of IgG titers [4]. This overestimation can result in increased treatment costs due to use of immunosuppressants, therapeutic plasma exchange and an increased hospital stay. So, to determine the actual concentration of IgG antibodies, the IgM antibodies need to be inactivated. Several methods have been described in the literature to inactivate IgM. These methods include heat inactivation at 63 °C in addition to the use of sulfhydryl reagents such as 2-mercaptoethanol (ME) and dithiothreitol (DTT) [10,11]. Heat inactivation of plasma has several advantages over DTT treatment such as less time consuming, no dilution of the sample and avoidance of the use of chemical agents. But the effects of heat are less specific than DTT as only heat sensitive proteins are denatured. The effects of heat are relatively less on IgG due to which they are minimally affected [12].
Measurement of ABO antibodies is routinely performed by titration which is a semi quantitative method. Titration can be performed by different techniques which include conventional test tube technique (CTT) that has been used widely for long. However, there are a number of limitations of this technique such as intensive labour and more time consumption, low reproducibility, inter-observer and inter-laboratory variability. Other semi and fully automated methods of titration like column agglutination technology (CAT) and solid phase red cell adherence (SPRCA)/hemagglutination (HA) are now used by laboratories [13-16]. Many studies have compared CAT with CTT and have concluded that CAT has more advantages over CTT such as high reproducibility and objectivity as well as stable well- defined end points of agglutination reactions [14,17-20]. Fully automated methods such as SPRCA are also being widely used to increase the efficiency and productivity of testing [13-16]. While automation has the advantage of objectivity, ease of use and reproducibility with well-defined end points of agglutination reaction, standardization of these methods have still not been possible. There is significant variation between methods and laboratories [21-26]. The aim of the study was to compare the results obtained by HA/SPRCA with those obtained by CTT and CAT with use of heat treated plasma (pCTT, pCAT) by:
a) Calculating correlation between anti-A and anti-B (IgG and IgM) results of pCTT with 1+ strength of reaction, pCAT with 1+/2+/3+ strength of reaction as end point with results of HA/SPRCA.
b) Calculating and comparing median anti-A and anti-B (IgG and IgM) titers obtained by pCTT, pCAT and HA/SPRCA.
This was a prospective, observational study conducted in the department of Transfusion Medicine at a tertiary healthcare centre from October 2018 to March 2020. A sample size of 2000 was planned. Serum from each donor was treated with heat and titers were simultaneously tested by CTT and CAT. Untreated samples were tested by three methods; CAT, CTT and HA/SPRCA for anti-A and anti-B titers. All results were recorded for comparison.
All consecutive O blood group donors who were eligible to donate blood as per the guidelines laid down by the Drugs and Cosmetics Act, 1940 and the Standards for Blood Banks and Blood Transfusion Services were included in the study [27,28]. Pilot tubes collected at the time of donation were used for titration. After performing the routine testing, antibody titration was performed from the residual sample either on same day or on the next day of collection. If tested on the next day, the sample was stored at 4 °C. All donors who did not give consent to participate in the study, donors reactive for transfusion transmitted infections, samples with positive direct antiglobulin test or positive antibody screen were excluded from the study.
Heat inactivation of plasma was performed by the method described in ASHI Laboratory Manual [29]. Desired amount of plasma was taken in a test tube and was placed in pre-heated 63 °C heat block for exactly 13 minutes using a stop-watch. After completion of 13 minutes, the tube was removed from heat block and was centrifuge. The supernatant was removed into another labelled test tube and was used for titration by CTT and CAT. The heat-treated sample was used on the same day.
CTT: Titration was performed by CTT according to the method described in AABB technical manual [4]. The titer end point was the reciprocal of the lowest dilution yielding 1+ agglutination with naked eye. The reactions were recorded and recorded for IgM and IgG on a case reporting form.
CAT: For IgM titer, Neutral Ortho BioVue System cassettes (Ortho Clinical Diagnostics, Raritan, New Jersey, USA) were used while for IgG, Anti-IgG Monospecific Ortho BioVue System cassettes (Ortho Clinical Diagnostics, Raritan, New Jersey, USA) were used. Dilutions of test sample were prepared as for CTT and testing was performed as per manufacturer's instructions. The reactions were then read and recorded on the case reporting form. The titer end point was the lowest dilution yielding 1+, 2+ and 3+ agglutination visible to the naked eye.
Antibody titration on neo immuohematology analyzer (automated method) by HA/SPRCA: IgM titers were performed by hemagglutination technique and IgG titers were performed by solid phase red cell adherence technique as per manufacturer's instructions. Agglutination reactions were read by automated cameras. The titer endpoint was the reciprocal of the lowest dilution yielding 1+, 2+ and 3+ agglutination.
To reduce inter-observer bias for manual (CTT) and semi-automated (CAT) methods, each sample was given to two different personnel to perform the test. Results obtained were given to the Transfusion Medicine physician. Final results were declared by the Transfusion Medicine physician.
Data was entered in an MS excel sheet; numerical values, percentages, mean and standard deviation was calculated. Statistical analysis was performed using SPSS software (Version 25.0.0.0, Chicago, USA). Median IgM and IgG titres were calculated for anti-A and anti-B obtained by pCTT, pCAT and HA/SPRCA at three different end points (1+, 2+ and 3+). Correlation between the methods was tested using Spearman's rho for the first 200 samples only. The strength of the correlation was calculated using the following guide for the absolute value of rs:
0.0-0.19 - very weak
0.20-0.39 - weak
0.40-0.59 - moderate
0.60-0.79 - strong
0.80-1.0 - very strong
Nonparametric Wilcoxon signed-rank paired test was used to test for significance comparing IgM and IgG results between pCTT(1+) and pCAT(1+), pCTT(1+) and HA/SPRCA, and pCAT and HA/SPRCA for a given sample. For this purpose, a total of 10 samples (every 200th sample) were included.
A total of 2005 O blood group, healthy, whole blood donors participated in this study out of which 1916 (95.5%) were male and 89 (4.4%) were female. Mean age of the participants was 32.2 ± 8.05 years. A to A total of 158 (7.88%) samples had inter-observer variation by CTT and 33 (1.65%) samples had inter-observer variation by CAT. Figure 1 illustrates the distribution of anti-A and anti-B titers, IgG and IgM titers performed by pCTT and HA/SPRCA with 1+ strength as end point with the help of box and whisker plot. From Figure 1a and Figure 1b, it is evident that while pCTT IgM results are higher than HA, pCTT IgG results are similar or lower when compared to results obtained by SPRCA. However, from Figure 1c and Figure 1d, it is evident that pCAT IgG and IgM titers were higher with pCAT when compared to HA/SPRCA and this difference was more evident with IgG titers.
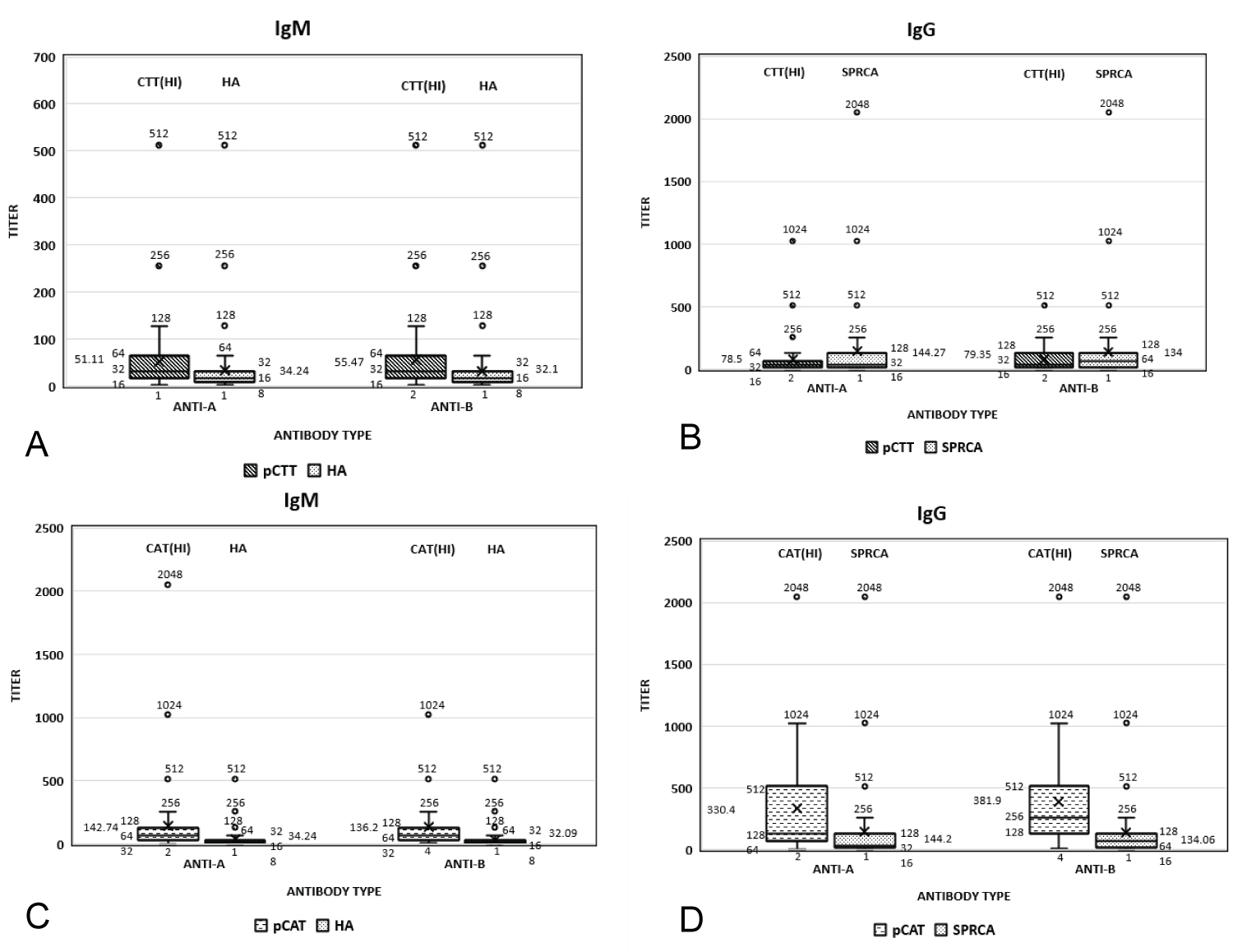 Figure 1: Distribution of Anti-A and Anti-B titers. a) Igm titers performed by Pctt and HA; b) Igg titre performed by Pctt and SPRCA; c) Igm titers performed by Pcat and HA; d) Igg titers performed by Pcat and SPRCA.
View Figure 1
Figure 1: Distribution of Anti-A and Anti-B titers. a) Igm titers performed by Pctt and HA; b) Igg titre performed by Pctt and SPRCA; c) Igm titers performed by Pcat and HA; d) Igg titers performed by Pcat and SPRCA.
View Figure 1
Figure 2 shows a comparison of distribution of measured IgG and IgM ABO isoagglutinin titer results obtained by pCTT, pCAT and HA/SPRCA at 1+ strength end points. IgM titers were found to be lower than IgG titers. Results of titers performed by pCAT were higher than results obtained by HA/SPRCA and pCTT. Results of titers obtained by pCAT across all categories were higher as compared to pCTT and HA/SPRCA, with maximum number of samples giving results more than 32. Results of titers obtained by pCTT across all categories were lower as compared to pCTT and HA/SPRCA, with maximum number of samples giving results less than 64.
 Figure 2: Comparison of distribution of measured IgG and IgM ABO isoagglutinin titer results obtained by pCTT, pCAT and HA/SPRCA at 1+ strength end points.
View Figure 1
Figure 2: Comparison of distribution of measured IgG and IgM ABO isoagglutinin titer results obtained by pCTT, pCAT and HA/SPRCA at 1+ strength end points.
View Figure 1
Figure 3 shows the comparison between median IgG and IgM titers for anti-A and anti-B by pCTT, pCAT and HA/SPRCA with results interpreted at 1+, 2+ and 3+ end points. Median IgM titers for anti-A and anti-B were found to be lower than median IgG titers. Median IgG and IgM titers for both anti-A and anti-B were highest in pCAT, while median IgG and IgM anti-A and anti-B titers were similar with HA and pCTT. Median anti-A and anti-B titers were when performed by all three methods.
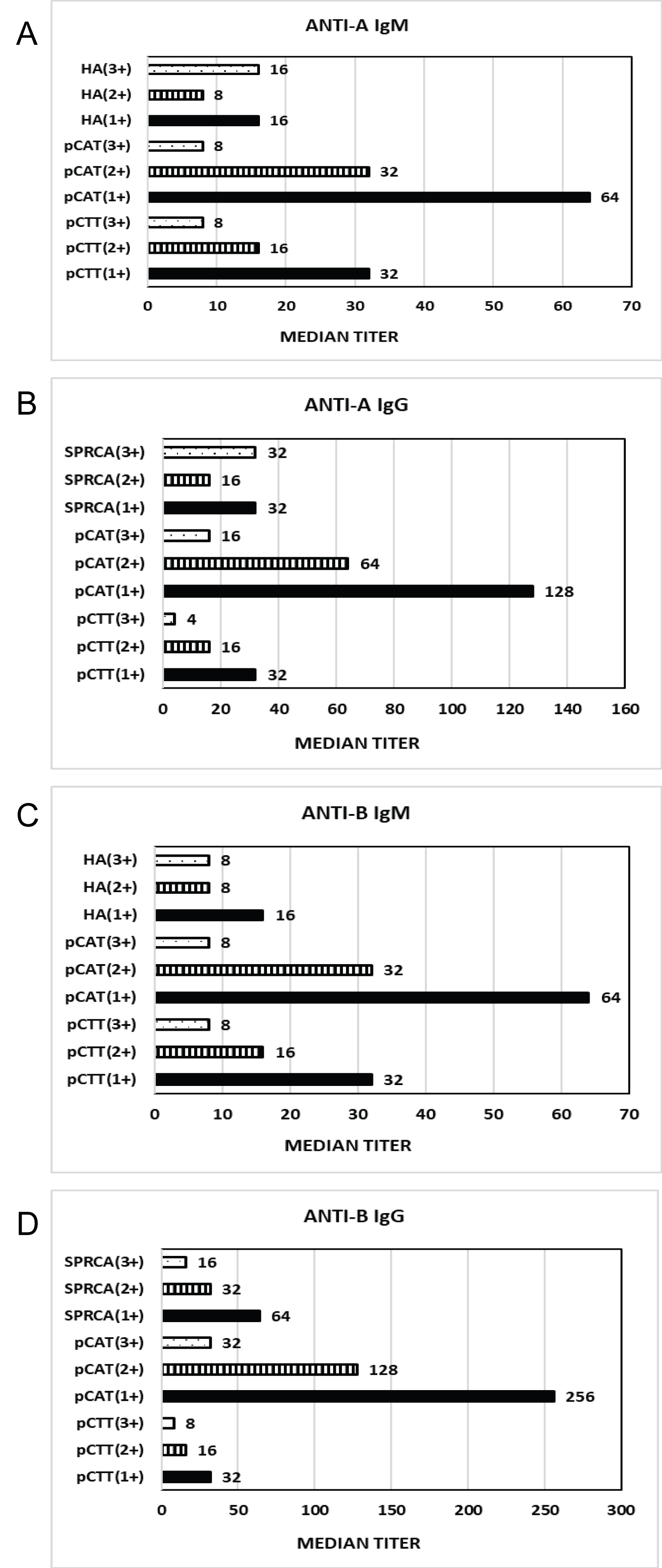 Figure 3: Comparison of median IgG and IgM titers for anti-A and anti-B by pCTT, pCAT and HA/SPRCA with results interpreted at 1+, 2+ and 3+ end points.
View Figure 3
Figure 3: Comparison of median IgG and IgM titers for anti-A and anti-B by pCTT, pCAT and HA/SPRCA with results interpreted at 1+, 2+ and 3+ end points.
View Figure 3
Table 1 lists the Spearman's rho (rs) for correlation between pCTT (1+ strength) and HA/SPRCA as well as between pCAT (1+, 2+ and 3+ strengths) and HA/SPRCA for the first 200 samples. The statistical analysis was performed for IgM and IgG titers for both anti-A and anti-B antibodies individually. The results show that for IgM as well as IgG, both anti-A and anti-B showed moderate correlation between pCTT (1+ strength) and HA/SPRCA. Similarly, for both IgM and IgG, anti-A as well as anti-B showed moderate correlation between pCAT (1+, 2+ and 3+ strengths) and HA/SPRCA.
Table 1: The correlation of results obtained by; a) SPRCA/HA and Pcat; b) SPRCA/HA and pCTT. View Table 1
Figure 4 illustrates the trend of IgM and IgG results obtained for every 200th sample by all three methods. A comparison between pCTT (1+) and pCAT(1+), pCTT(1+) and HA/SPRCA and pCAT(1+) and HA/SPRCA was performed using Wilcoxon signed-rank paired test for significance. When comparing anti-A and anti-B IgG and IgM results, results of pCTT and HA/SPRCA were not found to be significant, whereas, results of pCAT and HA/SPRCA and results of pCTT and pCAT were found to be significant.
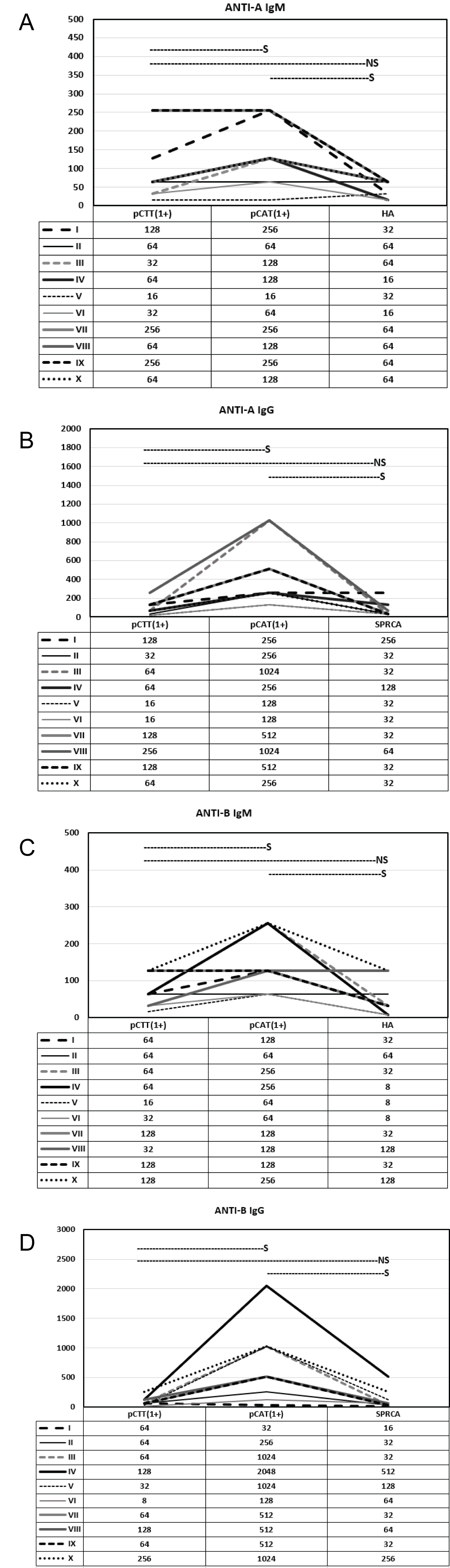 Figure 4: Comparison of IgM and IgG titers performed by pCTT at 1+ end point, pCAT at 1+ end point and HA/SPRCA for 10 samples. Wilcoxon signed rank test was used for calculating significance using p < 0.05. S indicates significant and NS indicates not significant. a) Anti-A IgM titers; b) Anti-A IgG titers; c) Anti-B IgM titers; d) Anti-B IgG titers.
View Figure 4
Figure 4: Comparison of IgM and IgG titers performed by pCTT at 1+ end point, pCAT at 1+ end point and HA/SPRCA for 10 samples. Wilcoxon signed rank test was used for calculating significance using p < 0.05. S indicates significant and NS indicates not significant. a) Anti-A IgM titers; b) Anti-A IgG titers; c) Anti-B IgM titers; d) Anti-B IgG titers.
View Figure 4
HA measures only IgM titres while SPRCA enables specific measurement of IgG and excludes IgM. Hence, IgM inactivation is not needed with HA/SPRCA; unlike CTT and CAT. ABO isohemagglutinin titration by CTT and CAT after the treatment of plasma by heat or by DTT is a time consuming procedure and needs expertise in comparison to the fully automated HA/SPRCA. In the present study, the authors compared the results of ABO titration by CTT and CAT post heat inactivation and HA/SPRCA.
The technique of heating plasma in order to inactivate IgM antibodies was first described in 1981 by Steinberg and Cook [30]. Studies performed previously for pretransplant work-up of solid organ transplant recipients have concluded that presence of IgM antibodies can cause false positive CDC crossmatch and subsequent use of heat inactivation technique can eliminate this false positivity by decreasing interference of IgM antibodies [31,32]. However, the effect of heat inactivation on the ABO isohemagglutinin titers have not been studied in detail when compared to study of the effect of DTT or 2-ME on ABO titers. Hasekura, et al. studied the effects of heat on IgG, IgM and IgA titers in 300 females with ABO incompatible fetus or newborns [33]. The present study was performed for 2005 healthy whole blood donors which included 1916 males and 89 females.
O blood group individuals are known to possess more IgG ABO isohemagglutinins as compared to A and B blood groups [25]. In the present study, IgG titers for anti-A and anti-B measured by both CAT and CTT were higher than IgM titers. When comparing 1+ strength of reaction, both IgM and IgG titers were found to be more when performed by CAT as compared to CTT.
Historically, heat inactivation of sera has been performed at 56 °C for 30 minutes to inhibit the complement activity [34,35]. Hasekura, et al. heated sera at 70 °C for 10 minutes and observed a significant decline in both IgM and IgG titers of anti-A and anti-B titers [33]. Riley, et al. heated plasma at 63 °C for 10 minutes to ameliorate the effect of IgM in false positive CDC crossmatch [32]. In the present study, HI was performed at 63 °C for 13 minutes as per ASHI laboratory manual [29]. Similar to Hasekura, et al., who expected more difference in titers according to the temperature, the authors of the present study did not observe a significant difference in anti-A and anti-B titers for both IgM and IgG types after HI of plasma [33]. In general, one-fold decrease in median titers was observed with HI of plasma. While reduction in titers does indicate that presence of IgM antibodies in samples leads to overestimation of IgG titers, this effect was quite evident when titers were performed by CTT whereas CAT results showed a modest decline only.
CTT has been the conventional method for all immunohematology investigations including ABO antibody titration, it is being replaced at various centers with other semi and fully automated techniques which do reduce the inter-observer variation and require much less expertise. However, these techniques are expensive and despite various advantages are not available to transfusion services in resource constraint settings. In the present study, the median IgG titers for both anti-A and anti-B were higher when performed by CAT in comparison to CTT while the median IgM titre for anti-A was similar between CTT and CAT but for anti-B, it was higher in CTT. Park, et al. compared only IgG titers of CTT with CAT and found that for blood group O, the titers were more in CAT than CTT. Median anti-A and anti-B titers by CTT for were found to be 32 in their study while in the present study, it was 8 [36]. Nayak, et al. compared results of 50 samples and concluded that there was poor agreement between IgG titers performed by CAT and CTT [37]. In the present study, positive correlation was obtained between HA/SPRCA and pCTT, pCAT. Lally, et al. compared titer results obtained by automated, solid-phase and agglutination-based antibody titer platform versus manual gel testing on 54 patient samples [38]. Of these 54 patient samples obtained for the study, 17 belonged to group O individuals. They found that in group O individuals, for anti-A and anti-B both, results obtained by CAT and HA/SPRCA were found to be significant. In the present study, when comparing anti-A and anti-B IgG and IgM results, results of pCTT and SPRCA were not found to be significant, while, results of pCAT and SPRCA and results of pCTT and pCAT were found to be significant.
HI helps in reducing the interference of IgM antibodies and its use is a simple, low cost method requiring simple equipment like a heat block or water bath which is available in most laboratories for IgM inactivation. However, it is slightly time consuming when compared to HA/SPRCA which does not require any treatment of plasma for IgM inactivation. Strengths of this study include a robust sample size of more than 2000 individuals and use of two different methods of titration, CTT and CAT. Limitations of the present study include non-specific denaturation of heat sensitive proteins, inability to study the clinical impact of titration performed after heat inactivation of plasma.
In conclusion, IgG titers were found to be higher than IgM titers when performed by all three methods. Results obtained by HA/SPRCA were closer to results obtained by pCTT, with the advantage of less time consumption, automation requiring less expertise and no inter-observer variation in comparison to pCTT or pCAT. Titers obtained by pCAT were higher in comparison to HA/SPRCA and pCTT results, probably due to high sensitivity. To discern the most suitable method, a clinical impact of these results needs to be studied.