Leukemia in children has a good prognosis with an overall cure rate of 85% in acute lymphoblastic leukemia [1,2] and 50-60% in acute myeloid leukemia [3]. Nevertheless in patients with refractory or relapsed leukemia the prognosis is limited and can only be cured by a salvage chemotherapy, in most cases followed by an allogeneic hematopoietic stem cell transplantation [4]. In this retrospective case cohort analysis we investigated the outcome of eight patients with relapsed or refractory acute myeloid (n = 2), lymphoblastic (n = 4), biphenotypic (n = 1) leukemia or T-lymphoblastic lymphoma (n = 1) who failed to respond to standard salvage regimens. They received a salvage therapy with melphalan and cytarabine at our institution between 2015 and 2019. After the salvage chemotherapy, 63% of the patients achieved a remission of the disease and qualified for subsequent allogeneic hematopoietic stem cell transplantation. The one-year overall survival rate was 50%, the three-year overall survival rate was 38%. 25% of patients experienced a temporary period of fever and SIRS. The reported results of our case cohort analysis indicate that a salvage therapy with melphalan and cytarabine in relapsed or refractory leukemia could represent a curative approach with the possibility of achieving remission and subsequent allogeneic hematopoietic stem cell transplantation.
Melphalan/Cytarabine, Pediatric leukemia, Refractory/Relapsed, Salvage therapy, Stem cell transplantation
AHSCT: Allogeneic Hematopoietic Stem Cell Transplantation; ALL: Acute Lymphoblastic Leukemia; AML: Acute Myeloid Leukemia; Ara-C: Cytarabine; BM: Bone Marrow; c-ALL: Common All; CAR: Chimeric Antigen Receptor; CR: Complete Remission; L-DN: Liposomal Daunorubicine; EFS: Event Free Survival; FLAG: Fludarabine Cytarabine G-CSF: Granulocyte-Colony Stimulating Factor; IDA: Idarubicin; Mel: Melphalan; MMFD: Mismatched Family Donor; MRD: Minimal Residual Disease; MUD: Matched Unrelated Donor; NA: Not Applicable; OS: Overall Survival; PR: Partial Remission; TF: Treatment Failure
In refractory or relapsed acute leukemia the achievement of a complete remission by a salvage therapy and the performance of allogeneic hematopoietic stem cell transplantation (HSCT) is in most cases the only treatment by which long term disease-free survival can be accomplished. With current salvage regimens a 5 year overall survival rate of 29% is obtained in children with relapsed acute myeloid leukemia (AML) and a 10 year overall survival rate of 36% in children with relapsed acute lymphoblastic leukemia (ALL) [3,5]. It has to be considered that children with relapsed leukemia have been exposed to high dose chemotherapy, especially if hematopoietic transplantation and thus intensive conditioning regimens have already been performed. Therefore a low toxicity regimen is needed. Melphalan is an alkylating drug with myeloablative and anti-tumor effect, which has been used in conditioning regimens prior to stem cell transplantation in different hematologic and non-hematologic malignancies [6-8]. The dose-limiting toxicity of melphalan is its bone marrow suppression, the extramedullary toxicity consists of mucosal damage, increase of liver enzymes and the development of interstitial pneumonia [6]. Cytarabine is an analogue of deoxycytidine. It is known to cause myelosuppression and gastrointestinal toxicity, fever and elevation of hepatic enzymes, neuronal toxicity and cardiac arrhythmia [4,9].
In Germany first line treatment for relapsed ALL and AML is conducted with an induction polychemotherapy, followed by consolidation therapy and an allogeneic stem cell transplantation in intermediate and high risk cases of relapsed ALL and in the majority of cases of relapsed AML according to the ALL-REZ-BFM 2002 protocol and the International Registry AML Relapse 2009 [4,10]. In case of second or third relapse an individualized chemotherapy with the introduction of new drugs is recommended [4].
In this retrospective case cohort analysis we investigated the outcome of a salvage therapy with melphalan and cytarabine in refractory or relapsed acute leukemia in children.
We describe a cohort of eight pediatric patients, which suffered from a primary refractory or relapsed acute leukemia and were treated with the salvage regimen of high dose melphalan and cytarabine. The patients were treated between the years 2015 and 2019 in the Children's University Hospital Tübingen. All patients were previously treated by a standard of care chemotherapy prior to high dose melphalan and cytarabine. Response was evaluated by examination of bone marrow (BM) before and after application of melphalan and cytarabine. In case of response, allogeneic hematopoietic stem cell transplantation was performed immediately afterwards. Additional supportive care was administered according to our institutional standards, including antibiotic, antifungal and antiviral prophylaxis.
A case was defined as primary refractory, if the patient had not responded to at least one previous standard of care chemotherapy.
A case was defined as relapsed, if the patient had initially achieved a morphologic complete remission after a standard of care chemotherapy and then had experienced a relapse of the leukemia (morphologic complete remission defined as < 5% leukemic cells in bone marrow). The event free survival (EFS) was defined as the time period from the first day of the first cycle of melphalan/cytarabine to the date of relapse, death or last follow-up.
The overall survival (OS) was defined as the time period from the first day of the first cycle of melphalan/cytarabine to the date of death or last follow-up. In case of death during the observation period the cause of death was subdivided into disease-related mortality and therapy-related mortality.
Treatment failure was defined as absence of complete remission without the possibility to proceed to allogeneic hematopoietic stem cell transplantation.
All medical reports derived from the SAP-system of the Children's University Hospital Tübingen (SAP Net Weaver SAP GUI for Windows, Version 7300.3.10.1084, SAP SE, Walldorf, Germany). The data were collected in Microsoft Excel (Microsoft Excel 2010 Version 14.0 for Windows, Microsoft Germany GmBH, Munich, Germany). Graphs were created with GraphPad Prism for Windows, version 7, 2017 (GraphPad Software Inc., La Jolla, CA, USA).
Patient characteristics are summarized in Table 1. A cohort of eight pediatric patients with primary refractory or multiply-relapsed acute leukemia was analyzed. The median age was 8.5 years (range 1 year - 18 years). Three patients had a primary refractory leukemia, including two patients with an acute lymphoblastic leukemia and one patient with acute biphenotypic leukemia. Five patients had a multiply-relapsed leukemia, including two patients with an acute myeloid leukemia, two patients with an acute B-cell precursor lymphoblastic leukemia and one patient with an acute T-lymphoblastic lymphoma. Six patients (75%) had a high risk cytogenetic constellation. All patients had received ≥ three regimens of chemotherapy before the melphalan/cytarabine therapy and four patients (50%) had received a prior allogeneic HSCT before the melphalan/cytarabine therapy. All patients had documented BM - involvement prior to the salvage therapy melphalan/cytarabine. Response was evaluated in BM before and after melphalan and cytarabine. In three of the eight cases (38%) only a remission in peripheral blood was documented between the salvage therapy and the allogeneic HSCT and a bone marrow puncture was performed after the HSCT. The aim of this practice was to minimize the amount of invasive procedures in order to reduce the pain and psychological stress in the children.
Table 1: Patient characteristics and outcome. View Table 1
The median dosage of melphalan was 1 × 40 mg/m2 with a range of 1 × 20 mg/m2 to 2 × 60 mg/m2. The reason for the low dosage of melphalan in one case was a high cumulative toxicity prior to the melphalan/cytarabine therapy. A very aggressive form of AML was the reason for choosing a higher dosage of melphalan. The median dosage of cytarabine was 3 × 1 g/m2 with a range of 5 × 100 mg/m2 to 4 × 1 g/m2. The reason for adopting a low dosage of cytarabine was high cumulative toxicity prior to the melphalan/cytarabine therapy. In this case cytarabine was given as a continuous infusion. Four patients (50%) received additional intrathecal chemotherapy. One patient (13%) received additional daratumomab. In this case the underlying disease was a CD38-positive T-lymphoblastic lymphoma. One patient (13%) with a common ALL received additional Anti CD19 antibody and one patient (13%) with AML received additional venetoclax. For further information concerning the melphalan/cytarabine regimen see Table 2.
Table 2: Dosages of the chemotherapy. View Table 2
Two patients (25%) experienced temporary high fever and severe inflammatory response syndrome (SIRS) after the melphalan/cytarabine administration prior to the allogeneic HSCT. No intensive care treatment was required. In one case (13%) a temporary elevation of liver enzymes GOT and GPT was registered (> eight fold the upper norm limit) after the salvage regimen melphalan/cytarabine. One patient (13%) developed a temporary severe disorder of blood coagulation requiring the administration of blood products and coagulation factors.
The median overall survival was 8 months (range 2 months - 75 months). Four patients (50%) survived at least one year (Figure 1). Four patients (50%) died within one year, all deaths were disease-related. Three patients (38%) survived at least three years (Figure 1). Five patients (63%) died within three years, four deaths were disease-related, one death was therapy-related (bacterial sepsis with subsequent renal and pulmonary failure) and occurred one year after Melphalan/Cytarabine. One patient died 3.6 years after Melphalan, the death was therapy-related (severe systemic Aspergillosis). Five patients (63%) achieved a remission and were able to receive an allogeneic stem cell transplantation afterwards. One of these patients developed chloromas after 4 months and was brought back into remission with blinatumomab, irradiation of the chloromas and a second stem cell transplantation. Two of these patients relapsed after 2 months and died shortly after. The other two patients did not relapse, but died later due to therapy-associated complications, as mentioned above.
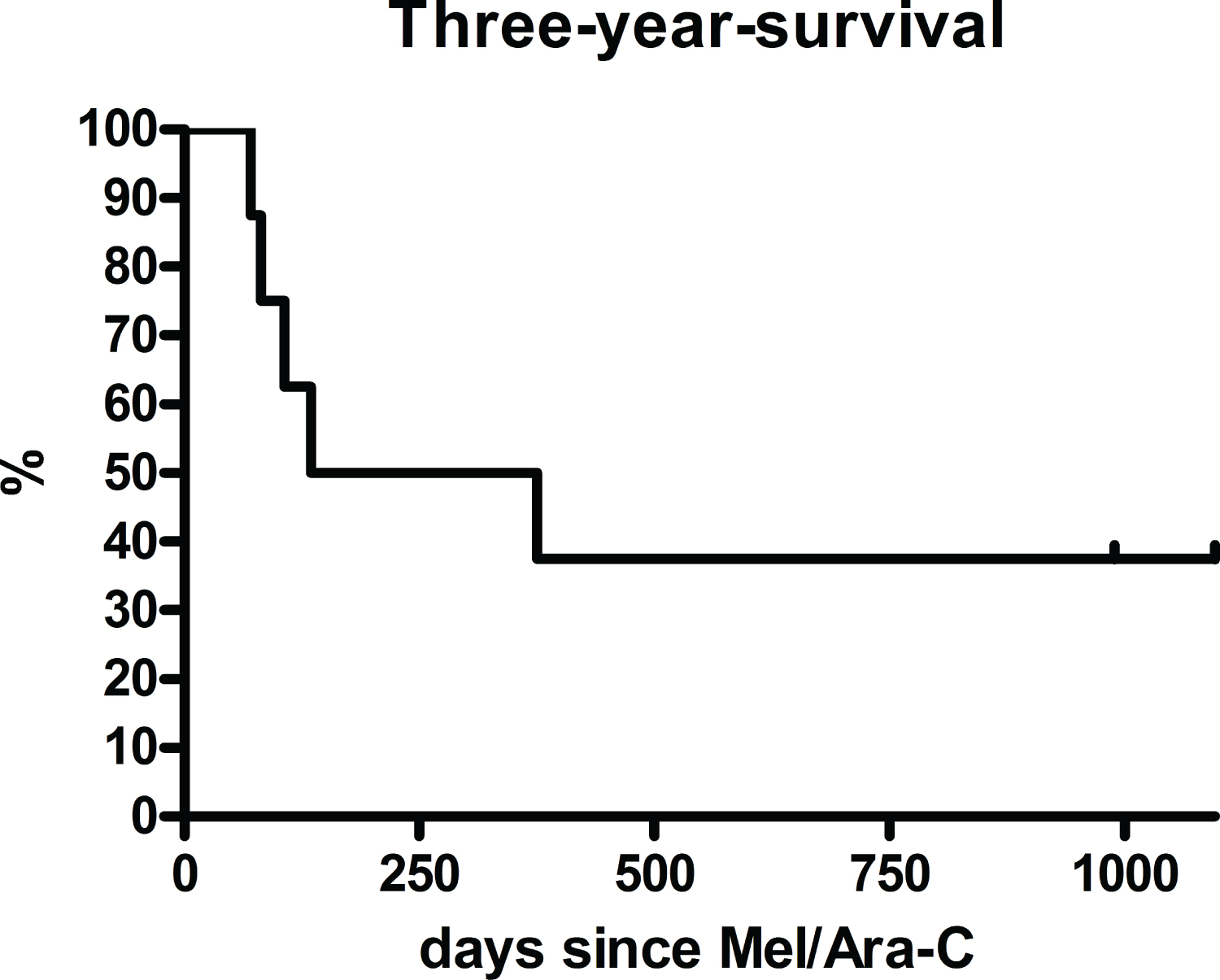 Figure 1: Overall survival rate of the whole cohort (n = 8) after a salvage therapy with melphalan and cytarabine with a one-year survival of 50% (n = 4) and a three-year survival of 38% (n = 3).
View Figure 1
Figure 1: Overall survival rate of the whole cohort (n = 8) after a salvage therapy with melphalan and cytarabine with a one-year survival of 50% (n = 4) and a three-year survival of 38% (n = 3).
View Figure 1
Three patients (38%) did not respond to the melphalan/cytarabine regimen and were considered as treatment failure. One patient (13%) received palliative care only. Two patients (25%) received another salvage regimen (consisting in one case of three cycles of clofarabine, cyclophosphamide and etoposide and in one case of one cycle of clofarabine, cyclophosphamide, etoposide and cytarabine). Both patients could be transplanted afterwards, one of these has since been in remission, 50 months after salvage therapy. The median event free survival was 4 months (range 2 months - 75 months).
In case of relapse or primary non-response to therapy, acute leukemias have a very poor prognosis. The aim in these cases is to achieve a remission by a salvage therapy and subsequently performing allogeneic hematopoietic stem cell transplantation. In case of a first relapse of AML in children, the chemotherapeutic drugs daunorubicine, cytarabine, idarubicine and fludarabine are used according to the International Registry AML Relapse 2009 [10]. In case of primary refractory or relapsed ALL in children cyclophosphamide, cytarabine, asparaginase, daunorubicine, corticosteroids, vincristine, methotrexate, idarubicine, thioguanine, ifosfamide are used according to the ALL REZ BFM 2002 protocol [4]. Currently different salvage therapies are tested with the aim to find a potentially curative therapy for the cases in which no complete remission can be achieved with standard chemotherapeutic regimens. In case of relapsed or refractory B-cell precursor leukemia the treatment with the immunomodulatory antibody blinatumomab could lead to a complete remission rate of 39% and a two year overall survival rate of 20% in a phase 1 - 2 multicenter study with 70 pediatric patients [11,12]. Recently the treatment with Chimeric Antigen Receptor (CAR)-T-cells is investigated in several studies. The use of these genetically modified immune cells in the therapy of relapsed/refractory CD 19-positive ALL resulted in a one-year overall survival rate of 76% in a phase 2 multicenter study including 75 pediatric or young adult patients [13].
The patients in our case cohort had been extensively pre-treated, all patients had received at least three regimens of chemotherapy before the salvage therapy with melphalan/cytarabine. All patients with ALL had received one or more cycles of blinatumomab. No patient had received a therapy with CAR-T-cells prior to the salvage therapy with melphalan/cytarabine, as they were not yet available. 50% of the patients had already received an allogeneic HSCT. The patients displayed a very high cumulative therapeutic burden. This is the reason why we chose a low dosage of melphalan (1 × 20 mg/m2) and cytarabine (10 × 100 mg/m2 as continuous infusion) in one case and in two cases we only reduced the dosage of cytarabine.
The reported toxicity in this case collection was low, 25% of patients experienced a temporary period of high fever and SIRS, no pathogen could be identified. One patient experienced a derangement of blood coagulation without other signs of SIRS. In one case a temporary increase of liver enzymes was reported. Cytarabine is known to cause temporary high fever, an increase of liver enzymes can be caused by melphalan as well as cytarabine [6,9].
The more chemotherapeutic regimens have failed to achieve a remission, the less likely achievement of a complete remission becomes. A possible explanation would be the formation of chemotherapy resistant leukemic clones. Accordingly, Gorman and his colleagues reported a complete remission rate of 17% in their retrospective study including 99 patients with multiply-relapsed AML following the fourth treatment attempt [3].
In primary relapse the response rates in AML and ALL are distinctively higher: In a retrospective study evaluating the outcome of children with primary relapsed or refractory acute myeloid leukemia a five-year survival rate of 34% was reported after a re-induction therapy with 6-thioguanine, cytarabine, doxorubicin, etoposide and mitoxantrone according to the NOPHO-88 an NOPHO-93 trials [14].
The use of fludarabine, high dose cytarabine, G-CSF (FLAG) and in some cases additional liposomal daunorubicin (L-DNR/FLAG) according to the International study Relapsed AML 2001/01 trial including 155 pediatric patients with primary relapsed AML and 10 pediatric patients with primary refractory AML lead to four year survival rates of 43% (L-DNR-FLAG) and 47% (FLAG) [15]. L-DNR/FLA without G-CSF is also recommended for patients with refractory ALL according to the AIEOP-BFM ALL 2009 protocol, leading to a MRD reduction < 5 × 10-4 in 50% of the cases [16].
A complete remission rate of 68%, a five-year overall survival rate of 24% and a toxicity rate ≥ grade 3 of 96% was reported in a retrospective single-center study including 53 pediatric patients with primary relapsed (68% of cases) or multiply-relapsed (32% of cases) AML or ALL after a salvage therapy with FLAG ± idarubicin (IDA) [17]. Keshu Zhou and his colleagues reported a two year overall survival rate of 30% in their study analyzing the outcome of patients with acute lymphoblastic leukemia in primary refractory cases (36%), in the first relapse (52%) or in the second relapse (11%) after a salvage therapy with granulocyte-colony stimulating factor (G-CSF), low dose cytarabine and aclarubicin [18].
The response rate in the present case collection is in line with the above mentioned studies: In 63% of our patients complete or partial remission could be achieved with the possibility to perform an allogeneic HSCT. The one-year survival and the three-year survival rates were 50% and 38%. It should be considered that it was the second or third relapse in three patients, the first relapse in two patients and three patients were primarily refractory and in addition all patients had received at least three previous therapy regimens.
Melphalan has so far mainly been used in conditioning regimens prior to stem cell transplantations. To our knowledge, it has not yet been used as a chemotherapy block with the aim of remission induction and subsequent conditioning for stem cell transplantation in primary refractory or multiply-relapsed AML or ALL. In the presented study we used melphalan as a salvage therapy in a median dosage of 40 mg/m² in one administration. Steckel, et al. reported an overall survival rate of 34% in primary refractory and 41% in relapsed AML in their study including 292 adult patients with AML. The patients in their study received a conditioning regimen with melphalan in a dosage of 140 mg/m2 in combination with fludarabine and a total body irradiation [19]. In another retrospective study the outcome after a conditioning regimen with melphalan in a dosage of 2 × 70 mg/m2 in combination with clofarabine and thiotepa and immediate subsequent stem cell transplantation was examined. This study included 18 pediatric and adult patients with AML or ALL in first or second relapse after an initial HSCT. The three-year overall survival was 49% and the therapy related mortality was 11% [20]. The results of our case cohort analysis indicate that melphalan could also lead to a cytoreductive effect in lower, non myeloablative doses, with the possibility of subsequent stem cell transplantation.
The present case cohort investigated the results of a salvage therapy with melphalan/cytarabine in eight children with primary refractory or multiply-relapsed acute leukemia. We registered a complete or partial remission in five patients (63%) with the possibility to proceed to allogeneic stem cell transplantation. Four patients (50%) survived at least one year; three patients (38%) survived at least three years after salvage therapy. Melphalan/Cytarabine in highly refractory acute leukemia might be a bridge to stem cell transplantation and in this context a potentially curative treatment. A future prospective trial should be conducted with higher patient numbers to verify these results and to identify differences in the response rates between various subgroups such as acute myeloid and acute lymphoblastic leukemias.
The authors report no conflict of interest.
ME, CIM and RH were responsible for the conception and design of this case cohort analysis. CIM collected, analyzed and interpreted the data. UH supported the data collection. ME, MQ, MD and PL have been involved in revising the manuscript critically for important intellectual content. All authors read and approved the final manuscript.
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.