The incidence of therapy-related Acute Promyelocytic Leukemia (t-APL) has been steadily rising. Radioactive Iodine (RAI) has emerged as a potential leukemogenic insult. Translocation involving the Retinoic Acid Receptor-alpha (RAR-alpha, RARa) gene on chromosome 17 and the Promyelocytic Leukemia (PML) gene on chromosome 15, denoted t(15;17), is pathognomonic of Acute Promyelocytic Leukemia (APL) leading to production of the classic PML/RARA fusion gene. This translocation interferes with the normal function of PML and RARa genes leading to abnormal proliferation of immature leukocytes that are in the promyelocytic stage. Trisomy of chromosome 8 is the most common additional genetic abnormality in t-APL with t(15;17), but cases of deletion in the long arm of chromosome 9 (9q deletion) have also been reported in de novo APL. Recent studies have suggested that 9q deletion in de novo APL is associated with poor overall survival. To our knowledge, 9q deletion in addition to the pathognomonic t(15;17) has not been described in t-APL. We report a case of interstitial deletion of 9q in a patient diagnosed with acute promyelocytic leukemia with prior exposure to RAI.
9q Deletion, Therapy related acute promyelocytic leukemia, Radioactive iodine
Therapy-related myeloid neoplasms listed in the 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia has emerged as a significant clinical entity in recent years [1]. This has been attributed to the increasing use of chemotherapeutic and radiotherapeutic agents for the treatment of primary neoplastic or non-neoplastic disorders, which can trigger treatment-induced mutagenesis. Although therapy-related Acute Promyelocytic Leukemia (t-APL) is a relatively infrequent disease, its steadily rising incidence has been associated with increasing use of topoisomerase II inhibitors [2,3]. Radioactive Iodine (RAI) therapy for thyroid cancer has also emerged as a possible leukemogenic insult. The balanced translocation between chromosomes 15 and 17, denoted t(15;17) is highly specific for Acute Promyelocytic Leukemia (APL). This translocation causes fusion of Retinoic Acid Receptor-alpha (RAR-alpha, RARa) gene on chromosome 17 and the Promyelocytic Leukemia (PML) gene on chromosome 15. Although several cases of t-APL have been identified, only six cases of t-APL have been reported after RAI therapy [4-9]. Deletion in the long arm of chromosome 9 (9q deletion) is a rare clinical finding that has been identified as a recurring cytogenetic abnormality in de novo APL and has been associated with poor overall survival [10-12]. Furthermore, 9q deletion with t(15;17) has not been previously described in t-APL after RAI therapy. We present a case of a woman who developed t-APL with interstitial 9q deletion after exposure to RAI therapy for her thyroid carcinoma.
A 60-year-old female was admitted to a hospital in August, 2016 with complaints of fatigue, nausea, vomiting, headache and epistaxis for one week. She had a prior history of papillary carcinoma of thyroid gland diagnosed in 2009 and treated with total thyroidectomy followed by 2 cycles of RAI therapy (cumulative RAI dose 202.5 mCi), and was taking sorafenib for locally recurrent disease at the time of admission. Initial laboratory workup was notable for severe thrombocytopenia with platelet count < 10,000/uL (normal 150-440 K/uL), fibrinogen level 38 mg/dL (reference range: 150-400 mg/dL), thrombin time 27.8 seconds (reference range: 10.3-16.6 seconds), D-dimer level > 20,000 ng/mL (reference range 0-240 ng/mL). Her peripheral smear had 22% blasts. As the patient was in a state of active Disseminated Intravascular Coagulation (DIC) with peripheral blasts, the decision was made to treat presumptively for APL with All-Trans Retinoic Acid (ATRA). She was admitted to the intensive care unit for close monitoring and further management.
Bone marrow aspirate and biopsy showed a hypercellular bone marrow with predominance of abnormal, hypergranular promyelocytes with variably shaped nuclei and numerous small to large cytoplasmic granules (Figure1). Occasional blasts with Auer rods, singly and in bundles, were further noted. A 500-cell differential cell count showed: 86% blasts (abnormal promyelocytes), 7% erythroid precursors, < 1% maturing myeloid precursors, 6% lymphocytes and 1% plasma cells. Flow cytometry (Figure 2) showed a prominent population of myeloid cells with dim CD45 expression and intermediate to high side scatter in approximately 95% of cells in the specimen. Blasts were positive for CD64 and partially expressed CD38, CD11b, CD13, and CD117. There was also evidence of partial, dim CD2 and/or CD56 expression in a minor subset of blasts. The blasts were negative for CD34 and HLA-DR. Cytogenetic analysis was performed on the bone marrow specimen, and a total of 20 cells were analyzed. Four cells showed a normal female karyotype. Sixteen cells showed an abnormal female karyotype with a translocation between the long arms of chromosome 15 (band q24) and chromosome 17 (band q21) with an additional chromosome 8 and interstitial deletion in the long arm of one chromosome 9 (bands q13q22) (Figure 3).
 Figure 1: a) Peripheral blood smear showing hypergranular blasts in our patient with t-APL (100x); b) Bone marrow aspirate showing leukemic promyelocytes, including one in the center (arrow) with Auer rods (100x). View Figure 1
Figure 1: a) Peripheral blood smear showing hypergranular blasts in our patient with t-APL (100x); b) Bone marrow aspirate showing leukemic promyelocytes, including one in the center (arrow) with Auer rods (100x). View Figure 1
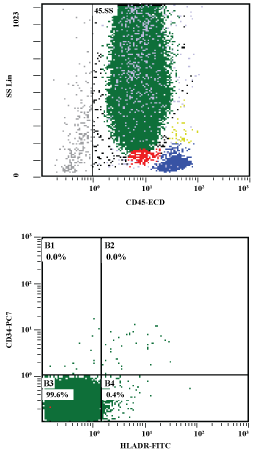 Figure 2: a) Note predominant blast cluster (green) with high Side Scatter (SS) CD 45 intermediate; b) Blasts are negative for CD34 and HLA-DR. View Figure 2
Figure 2: a) Note predominant blast cluster (green) with high Side Scatter (SS) CD 45 intermediate; b) Blasts are negative for CD34 and HLA-DR. View Figure 2
 Figure 3: Karyotype: 47, XX, +8, del (9) (q13q22), t (15;17) (q24;q21) [16]/46, XX[4]. View Figure 3
Figure 3: Karyotype: 47, XX, +8, del (9) (q13q22), t (15;17) (q24;q21) [16]/46, XX[4]. View Figure 3
The patient's DIC improved with aggressive supportive management and ATRA induction therapy. Her hospital course was complicated by differentiation syndrome, renal failure, respiratory failure, Escherichia coli bacteremia and septic shock. The patient continued to decline and passed away on day 6 of hospitalization.
RAI is a beta emitter and can cause chromosomal mutations that may be involved in the pathogenesis of therapy-related myeloid neoplasms. A meta-analysis including 16,502 thyroid cancer patients showed a 2.5-fold increase in the relative risk of developing leukemia in those patients who were treated with radioiodine [13]. Some of these inciting events can be identified using diagnostic cytogenetics. We identified 6 cases of t-APL following RAI therapy reported in the literature (Table 1). These patients had the pathognomonic t(15;17), but one report also documented a patient with trisomy of chromosome 8, which is the most common additional chromosomal rearrangement seen in APL. Of note, APL with trisomy 8 and PML/RARA fusion gene has been shown to have better prognosis compared to other AML subtypes with trisomy 8 [14].
Table 1: Reported cases of t-APL in patients treated with RAI therapy with underlying cytogenetic abnormalities. View Table 1
To our knowledge, 9q deletion has been described previously in 9 cases of de novo APL [11,15-18] but not in t-APL. Reported here is an unusual case of t-APL following RAI exposure with an interstitial deletion of 9q in addition to the pathognomonic t(15:17). Based on clinical observations, it has been proposed that APL with 9q deletion and the t(15:17) may have significantly worse prognosis than APL cases which have only the pathognomonic t(15;17) [11]. The unfavorable prognostic indication of 9q deletion has also been documented by an analysis of 51 cases of acute myeloid neoplasms with t(8;21)(q22;q22) [12]. Median overall survival of the patients with 9q deletion was reported as 12.5 months, significantly shorter than in patients with t(8;21) alone. These observations suggest that deletion of genes in this region of the genome may contribute to leukemogenesis and therapeutic resistance. Mutational analysis of the coding regions of genes in 9q has revealed a model of tumor suppression that can be disrupted by haploin sufficiency of critical genes due to 9q deletion, ultimately giving rise to myeloid leukemias [19]. Further research on the consequences of 9q deletion is warranted, in order to better understand how this genetic abnormality leads to poor prognosis and decreased survival.
A recent review of 37 cases of therapy related myeloid neoplasms following RAI therapy for thyroid cancer found a shorter latency period (1-4 years) in these patients when compared to those associated with alkylating agents and irradiation [9]. A short period of post-RAI treatment surveillance may be reasonable if a causative relationship is established between RAI exposure and development of myeloid neoplasms. Recent clinical studies have also investigated the benefits of radioprotective agents, both synthetic and natural, in mitigating genetic damage in patients undergoing RAI therapy [20].
Cases of t-APL have been reported to respond to treatment with good remission rates, but the presence of a 9q deletion has been associated with poor prognosis. Targeted therapies may be established in the future to improve survival in this subset of patients. Also, there appears to be an association between radioactive iodine treatment and this uncommon form of leukemia. Hence, awareness of this association by the treating clinician is important.
All authors attest that we have submitted for consideration for possible publication in the International Journal of Blood Research and Disorders a manuscript entitled: 'Interstitial deletion of chromosome 9q in therapy-related acute promyelocytic leukemia in patient exposed to radioactive iodine'.
We hereby certify that, to the best of our knowledge, the work reported in this manuscript has not received financial support from any commercial interest and none of the authors have any special financial interest in the subject matter described, with no exceptions.