Introduction: Glanzmann's thrombasthenia (GT) is a rare bleeding disorder caused by quantitative or qualitative deficiencies of the αIIbβ3 fibrinogen receptor on platelets. Platelet count and morphology are typically normal, but there is dysfunction in platelet aggregation. The global incidence is approximately 1 in 1,000,000 individuals. GT can lead to severe hemorrhages, particularly in post-traumatic or perioperative settings.
Objective: To describe the management of a case of GT diagnosed following intraoperative bleeding during mammoplasty and dermolipectomy.
Methodology: The study presents an epidemiological design of the descriptive observational type, involving analysis of the patient's medical records. Approval was obtained from the Human Research Ethics Committee. The case description adhered to the precepts outlined in the CARE Guideline.
Case description: A 33-year-old female Caucasian with non-consanguineous parents presented to a specialized hematology consultation with complaints of bleeding during mammoplasty and dermolipectomy. Laboratory tests, including a detailed coagulogram and platelet aggregometry, were performed. The results were conclusive for GT, leading to targeted management.
Discussion: The platelet light transmission aggregometry test serves as the gold standard for diagnosing GT. Early recognition of the condition is crucial to prevent severe bleeding episodes. Currently, there is no cure for GT, and treatment strategies vary based on the severity of the case. Platelet transfusion remains the most commonly employed method, while the use of activated recombinant factor VII (rFVIIa) shows promise in reducing both the frequency and severity of bleeding episodes.
Glanzmann's thrombasthenia, Platelet Glycoprotein IIb/IIIa Deficiency, Late Diagnosis, Case Report
Glanzmann's Thrombasthenia (GT) is a rare hereditary bleeding disorder characterized by platelet dysfunction [1]. It has an autosomal recessive inheritance pattern and a global incidence of approximately 1 in 1,000,000 individuals [2]. This condition involves quantitative or qualitative deficiencies of the fibrinogen receptor αIIbβ3 [3], resulting in normal platelet counts and morphology but decreased aggregation capacity [2].
According to the amount of αIIbβ3 expressed on the platelet membrane, GT can be classified into Types I, II, and III [4]. Clinically, GT can manifest as mucocutaneous bleeding, purpura, epistaxis, gingival bleeding, menorrhagia, and other symptoms [5]. Severe bleeding may occur in post-traumatic situations, surgical procedures, and tooth extractions [1].
For the diagnostic investigation of GT, a variety of tests can be utilized, including blood counts, coagulometric tests, platelet light transmission aggregometry (LTA), flow cytometry, and genetic mutation analysis [6]. GT typically shows absent or reduced platelet aggregation in response to physiological agonists, with normal agglutination in response to ristocetin [1,5].
There is no specific clinical treatment for GT [2]. For years, platelet transfusion was considered the gold standard [7]. However, due to the high risk of alloimmunization with repeated transfusions, the use of activated recombinant factor VII (rFVIIa) has emerged as a viable alternative [5,6].
This report presents a rare case of GT with a late diagnosis following intraoperative complications. The study was approved by the Human Research Ethics Committee under protocol 6,552,124 and adhered to the CARE Guideline. Its relevance is underscored by the rarity and the presence of associated complications of GT. This work aims to describe the clinical manifestations and the diagnostic methods employed to elucidate the case.
A 33-year-old female Caucasian patient with non-consanguineous parents experienced bleeding 17 days ago during mammoplasty and dermolipectomy. At the time, she required a platelet concentrate for intraoperative hemostasis.
The patient has a history of two pregnancies, both resulting in live births: one by natural delivery at 28 years of age and one by cesarean section 10 months ago. During the cesarean section, she experienced bleeding from pelvic varicose veins. At the age of 15, she underwent surgery to repair the anterior cruciate ligament of her left knee. This procedure was performed without any complications. Additionally, at the age of 18, she had four wisdom teeth extracted, also without any complications. The patient is allergic to Thimerosal (an organomercury compound) and nickel and is currently taking valerian (75mg/day). She denies any hospitalizations and signs of hemorrhagic predisposition or manifestations. She also reports no history of menorrhagia or metrorrhagia and has a negative family history of hemorrhagic diseases.
During a specialized hematological consultation, no abnormalities were found upon physical examination. She brought previous laboratory test results (Table 1), which did not indicate any bleeding disorder, showing only mild anemia following intraoperative bleeding. A new set of laboratory tests was requested, including a detailed coagulogram and platelet light transmission aggregometry test (Figure 1).
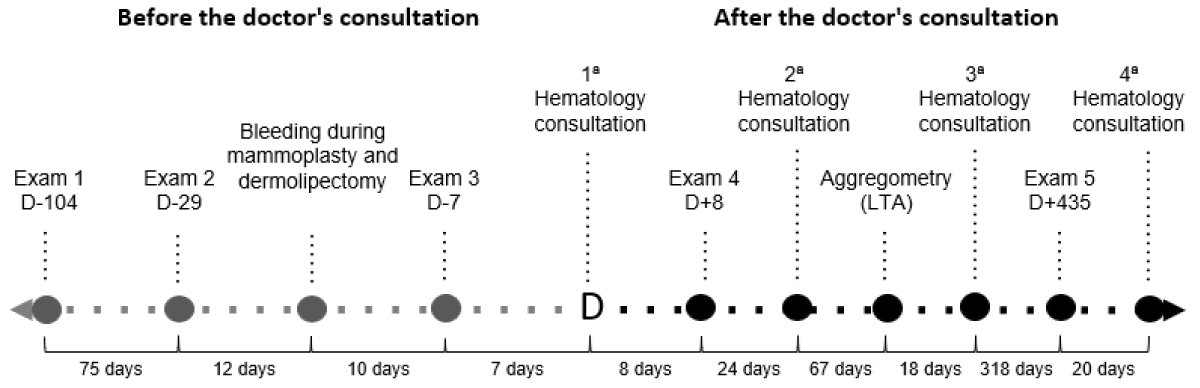 Figure 1: Timeline of diagnostic investigation.
Figure 1: Timeline of diagnostic investigation.
Legend: D: days relative to the first hematological consultation. LTA: Platelet light transmission aggregometry.
View Figure 1
Table 1: Laboratory results during clinical investigation. View Table 1
Upon return for reevaluation, analysis of the coagulogram results did not reveal any hematological abnormalities. The platelet light transmission aggregometry test provided a conclusive result for Glanzmann's Thrombasthenia (Table 2). She was informed about her condition, and it was determined that pharmacological treatment was not necessary. A new set of laboratory tests was requested for outpatient follow-up, which was evaluated in a subsequent appointment in the following year. No change in the test results was identified. Currently, she is stable, with no complaints, and is undergoing regular laboratory tests to monitor her condition.
Table 2: Platelet light transmission aggregometry results. View Table 2
Glanzmann Thrombasthenia (GT) is a rare hereditary bleeding disorder of platelet dysfunction [1]. The global incidence is 1:1,000,000 individuals [7]. People with GT have a high risk of bleeding events, especially in post-traumatic situations or during surgical procedures [1,8]. Although it is a highly relevant hematological syndrome, information about this condition, its clinical manifestations and therapeutic approaches are limited. This leads to medical inexperience in managing GT cases and results in variable health outcomes for patients.
Clinical manifestations arise from quantitative or qualitative deficiencies of the fibrinogen receptor αIIbβ3 (GPIIb/IIIa) on the platelet surface [1]. According to the amount of αIIbβ3 expressed, GT can be classified into Types I, II and III [4,9]. Type I is the most prevalent and presents < 5% of GPIIb/IIIa in the membrane; Type II shows between 5-20% and Type III has more than 20%, which may or may not be associated with dysfunction [1].
Hemorrhagic manifestations of GT include hematomas, purpura, epistaxis, gingival bleeding and menorrhagia [5]. The main bleeding complications occur after trauma and surgical procedures [1]. However, bleeding diathesis undergoes significant changes throughout life, independent of clinical severity, and exhibits an inverse relationship with age [9].
The available literature consistently points to a diagnosis established based on tendencies toward hemorrhagic manifestations. In this case, the patient did not show signs of hemorrhagic manifestations or predisposition to bleeding until adulthood. Prolonged bleeding was not observed during orthopedic surgery, tooth extraction or cesarean sections. The only known bleeding episode was during mammoplasty and dermolipectomy, which motivated the diagnostic investigation.
The diagnostic investigation carried out in the present case was in accordance with the Mexican Consensus for the Diagnosis and Treatment of Glanzmann's Thrombasthenia [6].
GT blood count typically shows normal platelet count and morphology, but with a decrease in aggregation [10], and it may be associated with iron deficiency anemia [9]. The tests conducted in the reported case support these findings. Before surgery, the patient had 208,000 platelets/mm³ and a hemoglobin level of 13 g/dL. During surgery, there was intraoperative bleeding, resulting in a hemoglobin level of 11.6 g/dL. Following the administration of platelet concentrate for intraoperative hemostasis, the platelet count rose to 308,000/mm³. This demonstrates that there was no coagulopathy due to platelet consumption.
Coagulometric tests, such as prothrombin time (PT), activated partial thromboplastin time (aPTT), thrombin time (TT), and fibrinogen levels, were normal, consistent with the literature on GT [5].
The clot retraction (CR) test, recommended to analyze platelet aggregation, typically shows minimal retraction in GT, while occlusion time (OT) results in prolonged durations (>300s) [6]. In this report, CR and OT tests were not performed.
Bleeding time (BT) can be used as an additional diagnostic strategy and is expected to be prolonged [6], but in our case the result was atypical, within the normal range (1 minute).
The platelet light transfer aggregometry (LTA) test is considered the gold standard for the diagnosis of GT [5]. In this test, centrifuged samples of platelet-rich plasma are monitored before and after the addition of a platelet agonist [9]. Platelet aggregation is absent or decreased in response to ADP, adrenaline, collagen, arachidonic acid, and thrombin; and normal to ristocetin [1,5,6]. This occurs because ristocetin induces platelet interaction through the GPIb-IX-V receptor complex instead of αIIbβ3 [6]. The results of the LTA in this report are consistent with a diagnosis of GT. The patient's values indicate hypoaggregation for ADP, adrenaline and collagen and normoaggregation for ristocetin.
GT can also be diagnosed through the analysis of genetic mutations [8]. This technology, often unavailable, involves high costs and its clinical application is limited. Flow cytometry is an alternative and quantifies the expression of GPIIb/IIIa on the platelet surface [6].
In this investigation, the diagnosis was made based on the LTA result. Due to unavailability and high cost, flow cytometry was not performed, making it impossible to determine the disease subtype. Given the absence of previous manifestations and bleeding tendencies, it can be inferred that this patient corresponds to GT Type III. Although we might reinforce the importance of following the diagnostic steps, the management of this case was not significantly limited by the lack of sub-classification.
The severity of bleeding guides the choice of treatment, along with the availability of drugs and previous responses to therapy [5]. Patients may not require continuous treatment, but may require intervention during surgical procedures and episodes of significant spontaneous bleeding [10].
For years, platelet transfusion was used as the gold standard in cases of severe bleeding or the need for a surgical procedure [6]. The risk of anti-GPIIb/IIIa or anti-HLA (human leukocyte antigen) alloimmunization after platelet transfusion is high [3].
rFVIIa is recommended for GT in patients with platelet antibodies and/or a history of platelet refractoriness. Its hemostatic mechanism is not fully known [2]. It is proposed that it activates Factor X, resulting in increased thrombin generation and platelet aggregation [1]. Its interaction through the GPIb-IX-V complex influences platelet activation in GT [6].
As there is no specific treatment for GT, each case must be individually evaluated [6]. In this patient’s case, drug treatment was deemed unnecessary, and expectant management was chosen. The only intervention was a platelet transfusion to control intraoperative bleeding. The patient continues to be monitored with annual laboratory tests, and progression to occasional bleeding tendencies is considered unlikely.
Although the patient has not experienced episodes of spontaneous bleeding, the risk of serious bleeding during future major surgeries is high. The perioperative use of platelet apheresis and rFVIIa in a patient known to have GT demonstrated operative success, without complications [2]. rFVIIa has been shown to be effective in surgical and non-surgical bleeding [6].
The limitations of the present study are the lack of initial LTA and tests to classify the GT subtype. As strengths, we have the description of an atypical case of GT, with late diagnosis, conducted in adherence to the recommended diagnostic steps. We also present a discussion with a review of the main forms of treatment, highlighting the importance of individualized and preventive management.
Given the need to perform invasive procedures, the procedure must be carefully planned and it must be aligned between anesthesiologist, surgeon and haematologist [2]. Inexperience in the perioperative management of bleeding disorders is not uncommon. In this sense, patients with GT benefit from treatment in a center with experience and qualified staff [5].
Early diagnosis of Glanzmann Thrombasthenia (GT) is crucial for the prophylaxis of hemorrhagic manifestations. Timely identification can prevent severe bleeding during surgical procedures and allows for proper planning before undertaking high-risk interventions. It is vital to consider GT in the differential diagnosis of bleeding disorders. For diagnostic confirmation, light transmission aggregometry (LTA) must be performed. Advances in the treatment of GT are promising and can significantly reduce the frequency and severity of bleeding episodes. This case underscores that, even in adulthood, accurate recognition and management of GT can greatly enhance the quality of life and minimize major complications.