Background: Multiple myeloma is a potentially fatal hematological malignancy characterized by clonal growth of terminally differentiated B cells. The development of novel medicines (proteasome inhibitors, immunomodulatory medications, antibodies targeting cell surface molecules, and autologous stem cell transplantation) has improved patients' quality of life and overall survival rate with multiple myeloma. Despite the advancement of these new therapies that help resolve disease progression and hence provide remission during the induction phase, some patients still relapse, and others die from the disease itself or treatment-related complications. Tanzania started treating patients with multiple myeloma using Melphalan and Prednisolone drug combinations more than 20 years ago. In 2017, a newer management protocol was implemented using MPT (Melphalan, Prednisolone, and thalidomide) and BTD (bortezomib, thalidomide, and dexamethasone) drug combinations. We have never evaluated our patient's responses and complications associated with using these drug combinations.
Objective: To determine the complications and response to treatment regimens among multiple myeloma patients attended at Muhimbili National Hospital.
Study population and methodology: A retrospective cohort study was carried out among patients diagnosed with multiple myeloma and managed at MNH from January 2017 to December 2020. A total of 177 medical records of patients from the registry of hematology patients at the medical record department of MNH and the Jeeva system were reviewed and analyzed. A systematic questionnaire was utilized to collect socio-demographic and clinical data. SPSS version 23 was used to perform statistical analysis on the data. P-values of less than 0.05 were considered statistically significant. To compare categorical data, Fisher's exact test was used. Continuous variables were analyzed using ANOVA for mean values and the Kruskal Wallis test for the median.
Results: In this study, 256 patients suspected of Multiple Myeloma attended from January 2017 to December 2020 were recruited. 89 patients were excluded; 70 following confirmation of other diagnoses, and 19 patients were lost to follow-up or had missing data. The vast majority of patients (n = 178, or 95.7 percent) reported bone pain and lytic bone lesions or fracture evidence. Most patients were in stage III, according to the Durie-Salmon Staging System. Of the 117 patients who received chemotherapy, 51% received BTD (Bortezomib, Thalidomide, Dexamethasone), 43.6% Melphalan and Prednisolone (MP), and 5.4% MPT. The number of chemotherapy cycles received was 4 for the patients kept on BTD and 6 for the patients kept on MP and MPT. The overall treatment response with complete remission was highest for BTD, followed by MPT. Those kept on the MP regimen had the lowest complete remission of about 20%. There was an increase in hemoglobin levels before and after treatment among patients presenting with anemia symptoms. A decrease in creatinine levels and a reduction in serum calcium levels were observed. There were twice as many deaths in those receiving the MP regimen than those on the other two-drug regimens. Peripheral neuropathy and cytopenia that involved mostly severe anemia and thrombocytopenia were the most reported and statistically significant complications of multiple myeloma therapy in this study.
Conclusion: The majority of patients received BTD as the treatment for myeloma. BTD regimen showed superior response compared to MP and MPT. The most common reported complication was peripheral neuropathy, with BTD having a reduced prevalence of other side effects.
Complications, Response, Treatment regimes, Multiple myeloma
BCNU: Carmustine; CRAB: C-Calcium level; R: Renal insufficiency; A: Anemia; B: Bone pain; DM: Diabetes mellitus; FLC assay: Free Light Chain assay; IMWG: The International Myeloma Working Group; IRB: Institutional Review Board; LDH: Lactate Dehydrogenase; MGUS: Monoclonal Gammopathy of Undetermined Significance; MM: Multiple Myeloma; MNH: Muhimbili National Hospital; MP: Melphalan-Prednisone; MRI: Magnetic Resonance Imaging; MUHAS: Muhimbili University of Health And Allied Science; VCD/BCD: Bortezomib(Velcade)-Cyclophosphamide-Dexamethasone; VTD/BTD: Bortezomib(Velcade)-Thalidomide-Dexamethasone; ER: Endoplasmic Reticulum; JNK: c-Jun NH2-Terminal Kinase; SPB: Solitary Plasmacytoma; HBT: Haematology and Blood Transfusion
Multiple myeloma is a B-cell malignancy characterized by clonal proliferation of terminally differentiated B lymphocytes (plasma cells). Plasma cells are essential components of the immune system that aid the body in fighting infections. MM is one of the numerous disorders known as plasma cell dyscrasias [1].
Multiple myeloma accounts for 1% of all cancers and roughly 10% of all hematologic malignancies. It is a treatable but incurable condition; thus, lifelong monitoring and follow-are advised [2-4].
It is anticipated that 120,000 new instances develop each year worldwide, and the incidence rate is expected to climb with the world’s aging population. The disease has a median age of 70 years, with a male predominance [5,6].
The cellular origin of multiple myeloma is thought to happen through transformation in lymphocyte within the germinal center (GC) of lymph nodes. During antigen encounter, naive B cells undergo class-switch recombination (CSR) and somatic hypermutation (SHM) of the B-cell receptor (BCR). Multiple myeloma develops from a pre-malignant condition characterized clinically as monoclonal gammopathy of unknown significance (MGUS). The exact mechanisms by which MGUS progresses to multiple myeloma are unknown. Multiple myeloma stages include an asymptomatic or subclinical phase before diagnosis (which can span many years), a chronic phase that lasts several years, and an aggressive terminal phase [2,7,8].
The classical form of Multiple Myeloma is distinguished by extensive neoplastic alterations in the bones, renal impairment, poor hematopoiesis, and susceptibility to infections. Multiple myeloma is not confined to a specific bone or location within a bone. It tends to involve the entire skeleton. When only one lesion is found it is called a plasmacytoma. Solitary plasmacytoma account for only 5% of malignant plasma cell tumors. They typically occur in the vertebrae or pelvic bones, with the presentation in the rib being less common. SPB are generally considered to be curable with surgical resection and radiotherapy. Complete resection is expected to be curative while subtotal resection should be followed by radiotherapy, and there is no role for chemotherapy if the patient has no evidence of multiple myeloma [5,6,9].
Multiple myeloma is detected in laboratory testing by the presence of a monoclonal protein termed paraprotein in serum or urine [5].
Criteria for the international myeloma working group (IMWG) symptomatic Multiple Myeloma diagnosis emphasized the significance of end-organ damage and laboratory results in determining the diagnosis. The next three requirements must be met: The presence of serum or urine monoclonal protein, plasmacytoma or bone marrow clonal plasma cells, as well as end-organ damage induced by plasma cell dyscrasias, such as elevated calcium concentration, lytic bone lesion, anemia, and renal failure [4].
The initial workup comprises a complete blood count, serum calcium, renal function tests (urea and creatinine), serum protein electrophoresis and immunofixation, urine for Bence Jones protein, bone marrow aspirate, trephine biopsy, and skeletal survey [3].
The international staging system (ISS), which uses serum beta 2 microglobulin and serum albumin levels to forecast disease stage and survival, is more widely used to find prognostic indications in Multiple Myeloma [4].
The Durie-Salmon Staging System, an older staging system, was used in this study. The Durie–Salmon Staging System determines the stage of myeloma based on four measurements: Hemoglobin level, blood calcium level, presence of bone lesions, and M protein synthesis rate [4,10]. Treatment options are determined by age, illness stage, stem cell transplant eligibility, performance status, and patient cost [3].
Treatment of MM involves the use of 2 to 3 drug combinations with autologous stem cell transplantation (ASCT). Drugs used include the alkylators, corticosteroids and novel therapies like proteasome inhibitors, immunomodulatory agents and monoclonal antibodies. Subsequently, thalidomide, bortezomib, and lenalidomide emerged as effective agents and greatly improved clinical outcomes [11-14].
Response to therapy or disease progression in MM is determined by either improved or worsening in biochemical indicators such as serum or urine M protein, FLC assays, bone marrow plasmacytosis, imaging scans for plasmacytoma and bone lesions.
Tanzania started treating patients with multiple myeloma using Melphan and Prednisolone drug combinations more than 20 years ago. In 2017, a newer management protocol was implemented using MPT (Melphalan, Prednisolone, and thalidomide) and BTD (bortezomib, thalidomide, and dexamethasone) drug combinations. Follow up clinic to see how the patient responds to a specific treatment regimen is usually done every month in routine clinical practice in our setting. It is done by measuring the level of serum M-protein using serum protein electrophoresis and by repeating the bone marrow aspiration procedure at the end of treatment course to see a reduction of plasma cells for those with non-secretory myeloma. Therefore, this study has evaluated the response and complications of all these drug combinations currently being used [2].
The aim of the study was to evaluate complications and response to treatment regimens among Multiple Myeloma patients attended at Muhimbili National Hospital. This was a retrospective cohort study involving all patients who were diagnosed with multiple myeloma at MNH from January 2017 to December 2020. 256 records denoted by the Jeeva System as MM were retrieved and screened for enrollment but only 117 patients were analyzed and reported in the period of 8 months (October 2021 to May 2022). Inclusion criteria included all patients who were diagnosed with multiple myeloma from January 2017 to December 2020 while patients who did not return for follow-up, those who had a lot of missing data that made analysis impossible, and those who did not choose treatment were excluded.
Patient details were recorded, such as age, sex, residence, insurance status, symptoms presented, and laboratory and imaging investigations results, including the M component. MM treatment regimen used and its complication, if any, was also recorded. Finally, follow-up clinic notes were reviewed on the Jeeva system to gather information on the number of treatment cycles received, M component, haemoglobin level, Creatinine level, and calcium level during each visit. The median follow up time for patients on BTD was four (4) months while those who received MP and MPT was six (6) months.
The statistical package software for social sciences (SPSS) version 23 was used for data entry, validation, and analysis. Frequency distribution tables and figures were generated for the categorical variables. Tests of significance were generated for different variables. Data were examined for normality. A P-value of less than 0.05 was used to denote statistical significance. Fisher’s exact test was used for the comparison of categorical variables. Continuous variables were analyzed using ANOVA for mean values, and the Kruskal Wallis test was used for the median.
In this study, 256 records denoted by the Jeeva System as MM were retrieved and screened for enrollment. Following the initial screening, 186 were confirmed from the records to have MM based on clinical presentation, laboratory findings, and imaging, so they were enrolled in the study. Seventy patients were confirmed with other diseases and not multiple myeloma. 19 patients were lost to follow-up, 37 had a lot of missing data impossible for analysis, and 13 did not opt for treatment. Only 117 patients were analyzed (Figure 1).
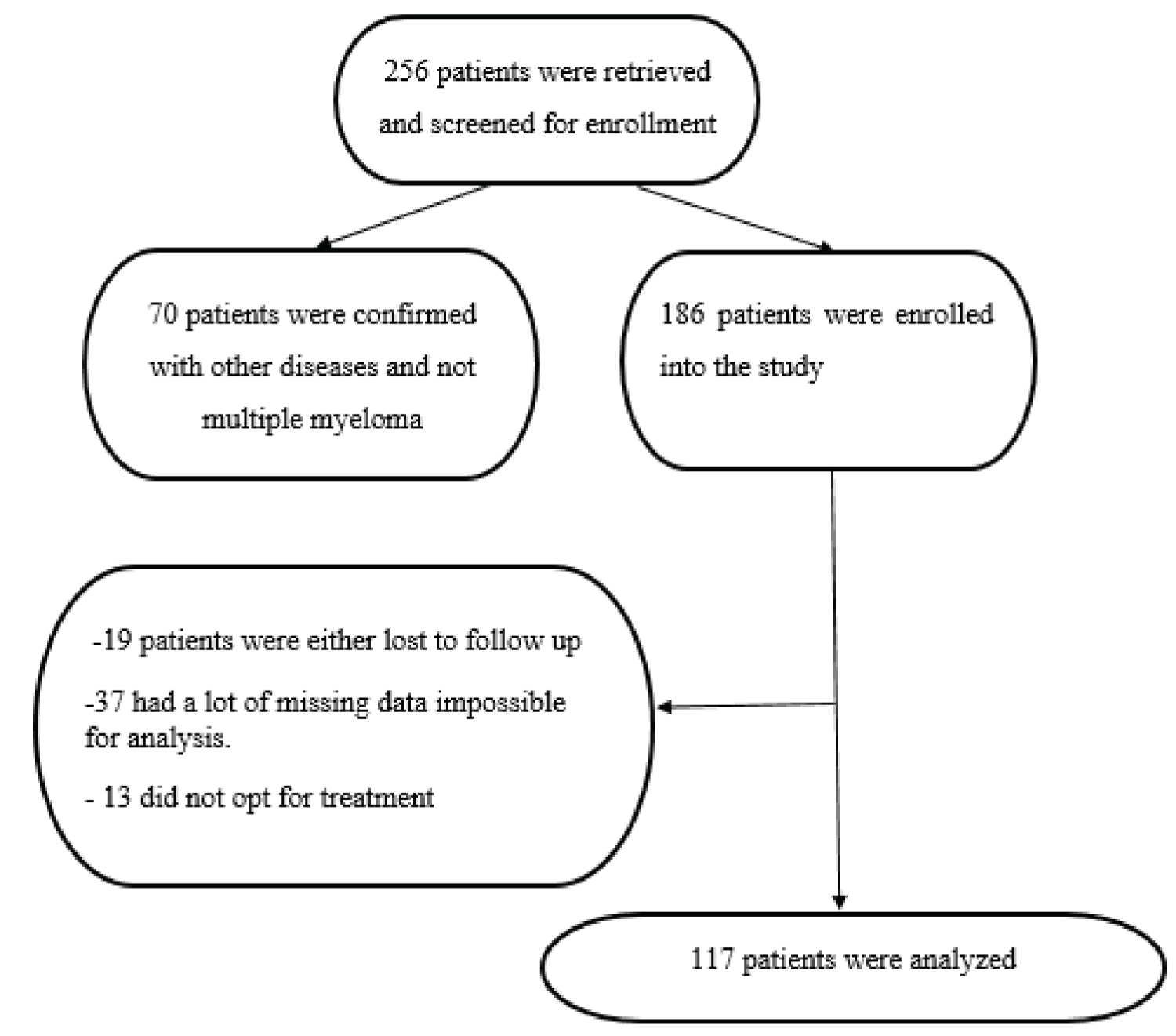 Figure 1: Flow chart of participants' enrolment.
View Figure 1
Figure 1: Flow chart of participants' enrolment.
View Figure 1
The median age of all study participants is 62 years. Majority were males and accounted for 53.1% (95/186) of the study sample. Many patients (n = 178, 95.7%) presented with bone pain and had lytic bone lesions or evidence of fracture. 81% (146/186) of the participants were employed. More study participants had health insurance making up 66.1% (117/186) of all study participants. According to Durie-Salmon Staging System, most study participants were in stage III (47.1%, n-81), followed by stage II (43.6%, n-75) (Table 1).
Table 1: Socio-demographic and clinical characteristics of the study participants. View Table 1
Figure 2 illustrates 186 patients who received chemotherapy. The majority, 51% (95/186), received BTD (Bortezomib, Thalidomide, Dexamethasone), followed in descending order by 43.6% (81/186) Melphalan and prednisolone (MP) and 5.4% (10/186) MPT (Melphalan, Prednisolone, Thalidomide). The chemotherapy cycles received were 4 for BTD and 6-8 for MP and MPT. Therapy was stopped or changed only if the patient presented with unbearable side effects of the therapy or confirmed with treatment failure (Figure 2).
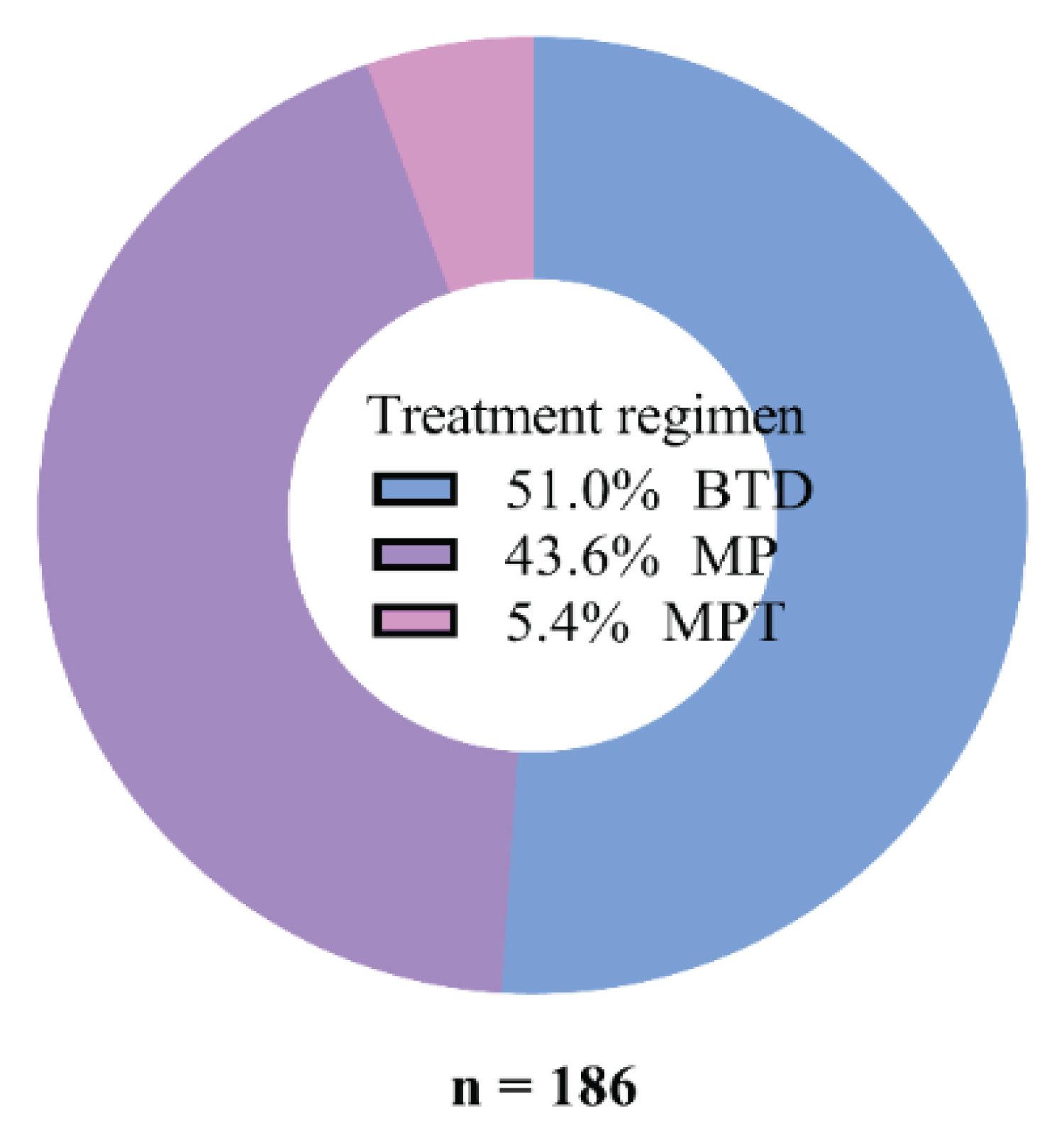 Figure 2: Common treatment regimens used for MM at MNH.
View Figure 2
Figure 2: Common treatment regimens used for MM at MNH.
View Figure 2
The BTD regimen had the highest proportion of participants attaining complete remission at 45% (27/60), followed by MPT at 33% (2/6) and MP at 20% (10/51) (Figure 3).
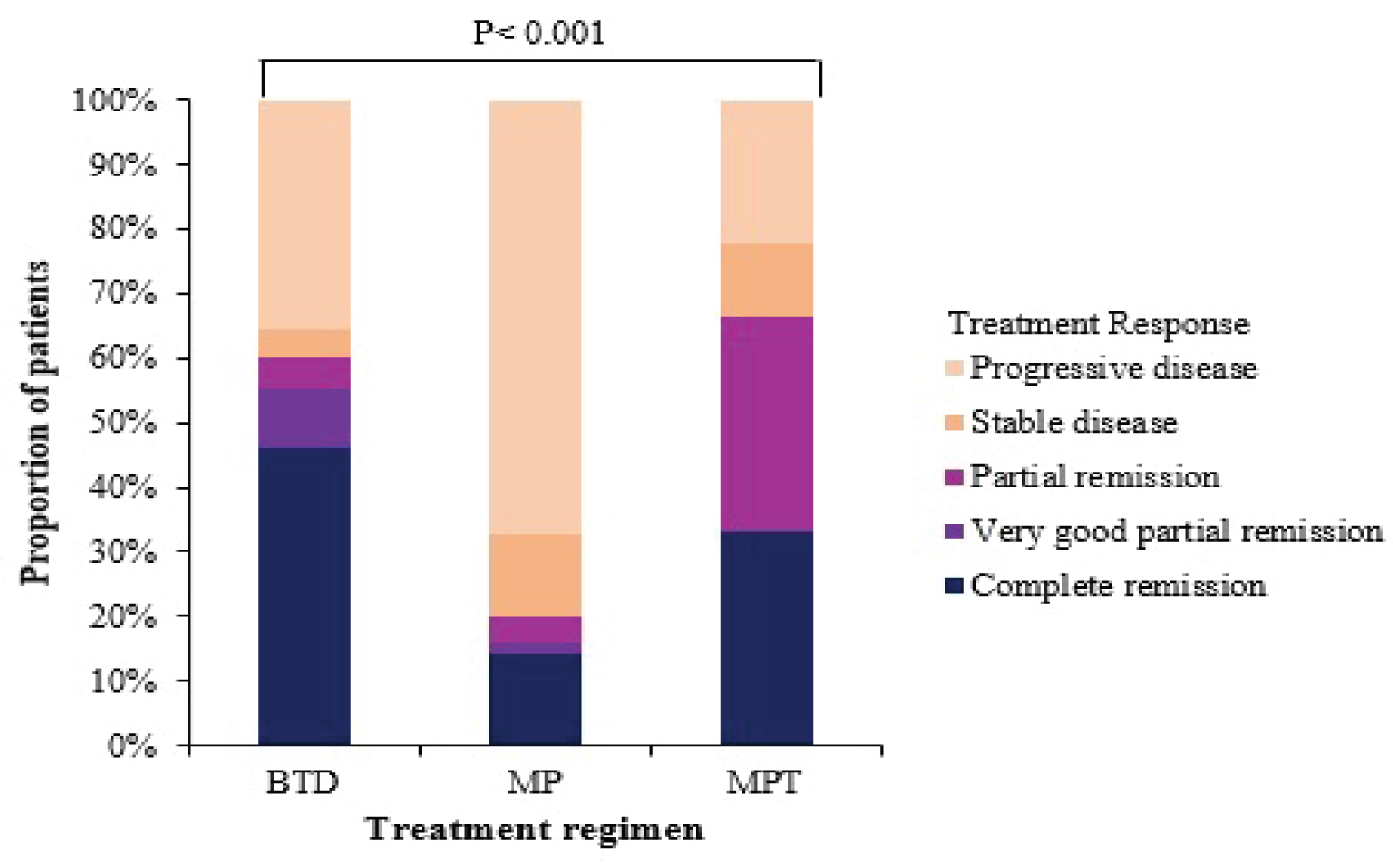 Figure 3: Response to treatment regimens given at MNH for MM.
View Figure 3
Figure 3: Response to treatment regimens given at MNH for MM.
View Figure 3
Upon comparing the Hb level before and after treatment, an increase in the Hb level was observed with all three regimens for participants presenting with symptoms of anemia and a low Hb. The BTD regimen had the highest increase in hemoglobin level with the mean Hb increase of 1.38 g/dl, followed by 0.79 g/dl and 0.63 g/dl with MP and MPT. However, these differences between individual means across the treatment groups were not statistically significant, as indicated in Table 1 and Table 2. Furthermore, Figure 4 shows the forest plot comparing the change in Hb level among patients with multiple myeloma receiving different treatment regimens.
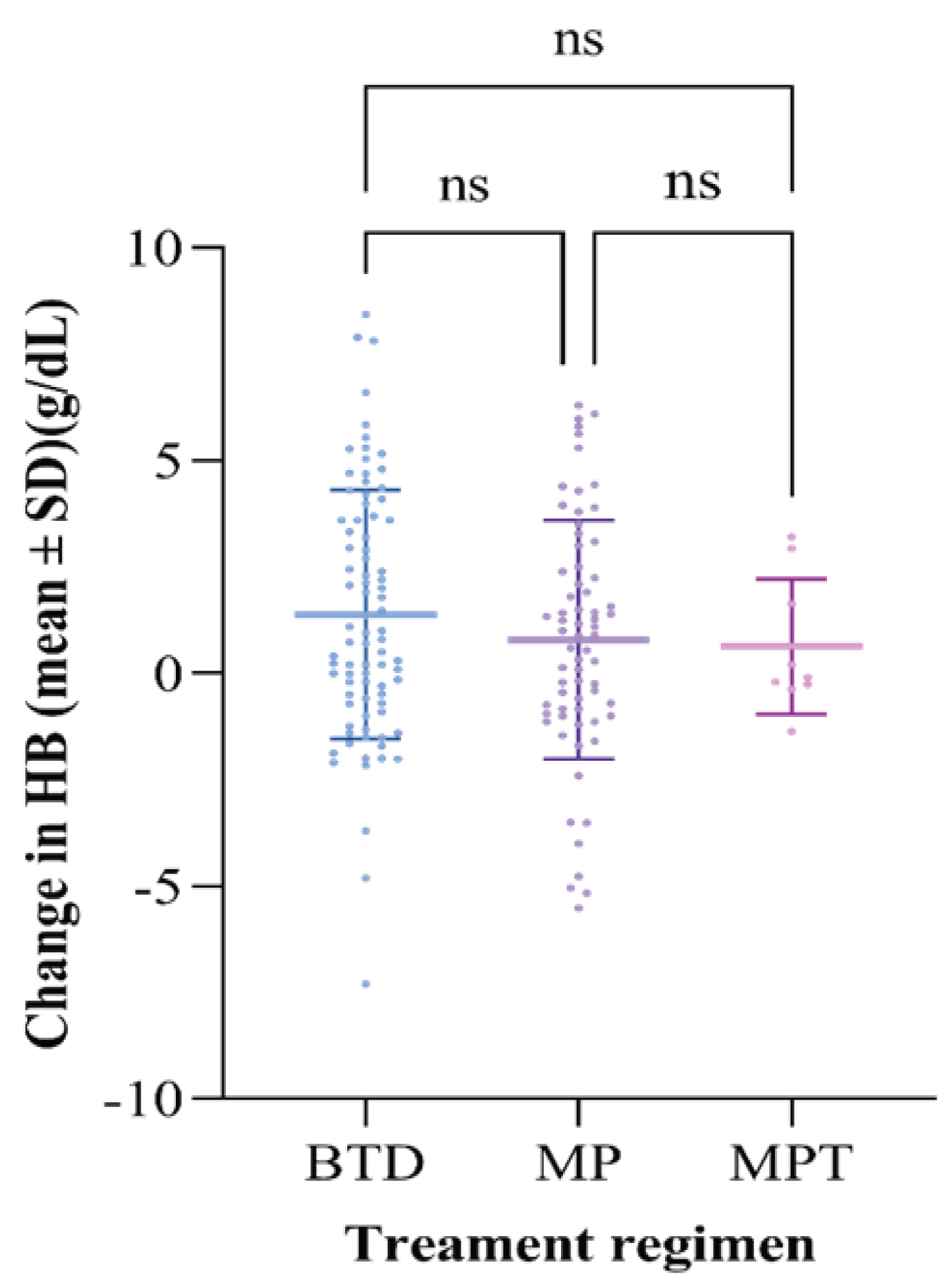 Figure 4: Forest plot comparing the change in Hb level among patients with multiple myeloma receiving different treatment regimens.
View Figure 4
Figure 4: Forest plot comparing the change in Hb level among patients with multiple myeloma receiving different treatment regimens.
View Figure 4
Table 2: Change in laboratory parameters before and after treatment. View Table 2
Whereby:
• ns means not statistically significant
• Each point denotes a change in the Hemoglobin of an individual patient
• Upper line and lower line (interquartile range)
Median was used to analyze a decrease in serum creatinine and calcium levels before and after treatment because the data was not normally distributed. A reduction in the serum creatinine and calcium levels was observed in all the treatment regimens. However, there was no statistically significant difference between the regimens in these observations, as Table 2 and Figure 5 illustrate.
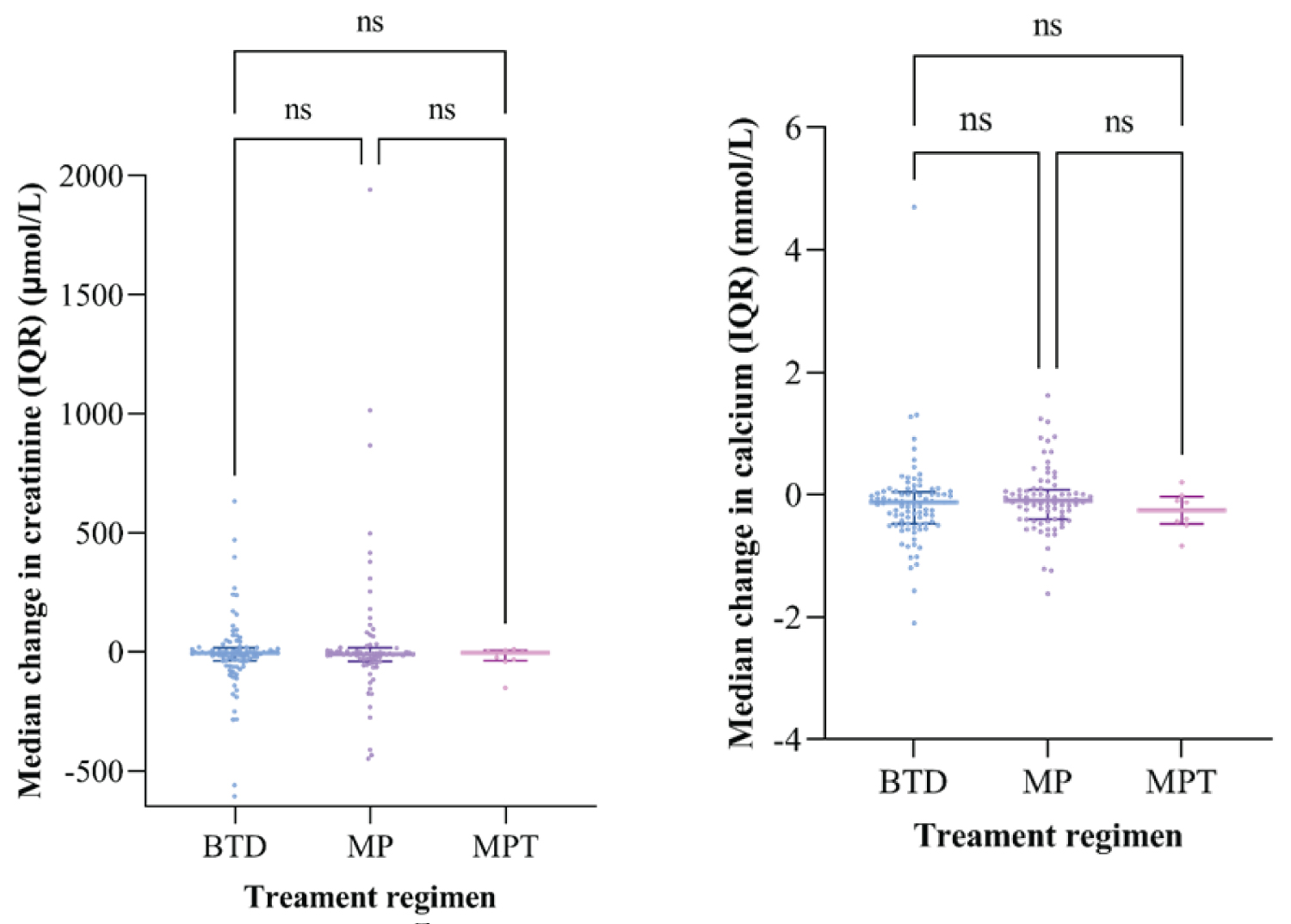 Figure 5: Forest plot comparing creatinine level and serum calcium levels among patients with multiple myeloma receiving different treatment regimens.
View Figure 5
Figure 5: Forest plot comparing creatinine level and serum calcium levels among patients with multiple myeloma receiving different treatment regimens.
View Figure 5
Whereby:
• ns means not statistically significant
• Each point denotes the median change in serum creatinine/ serum calcium of an individual patient Upper line and lower line show an interquartile range
Table 3 and Figure 6 below illustrate the following observations;
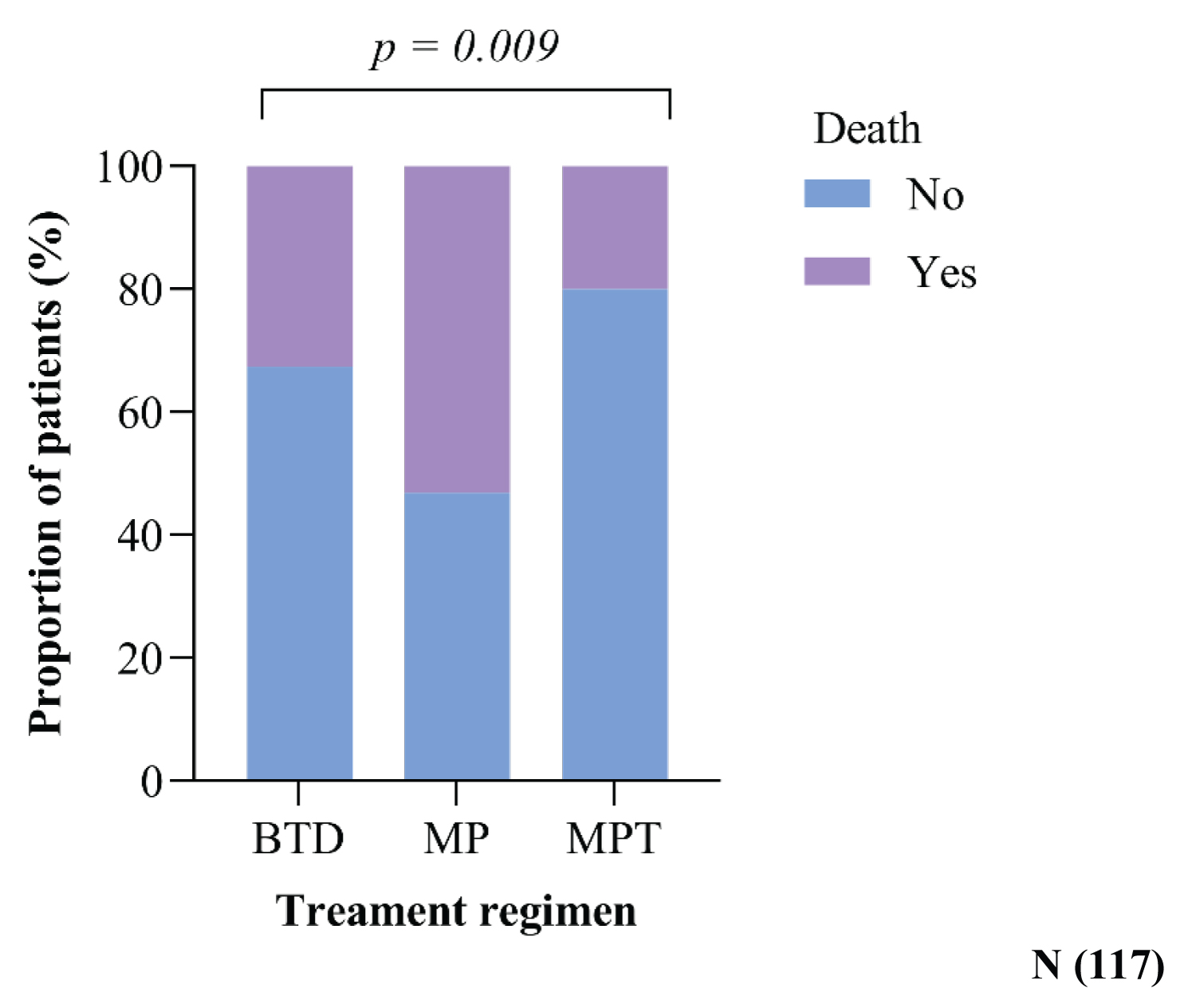 Figure 6: Complications Associated with Treatment Regimen given at MNH for MM.
View Figure 6
Figure 6: Complications Associated with Treatment Regimen given at MNH for MM.
View Figure 6
Table 3: Statistics showing complications associated with treatment regimen given at MNH for MM. View Table 3
The presence of peripheral neuropathy was significantly higher in patients on BTD 45/93 (48.4%) compared to those on MP and MPT 5/77 (6.5%) and 4/9 (44.4%) of study participants in each group, respectively.
Cytopenia mainly included severe anemia and thrombocytopenia, which were not there before treatment or worsened after treatment. 18/77 (23.4%) of patients who received MP experienced this complication, followed by 1/9 (11.1%) of those who received MPT. Only 1/93 (1.1%) of study participants in the BTD regimen experienced this complication. These variations were statistically significant (p < 0.001).
The three regimens also observed gastropathy, edema, steroid-induced DM, and other complications like infections, eczema, hearing loss, long-standing blurred vision, and electrolyte imbalance. There was no statistically significant variation in the frequency of incidence among the different groups.
50% (39/77) of participants died in those who received the MP regimen because most patients in this group had progressive disease. The lowest death rate was observed in those who received MPT, i.e., approximately 20% (2/9) of participants (Figure 6).
Patients in their sixth and seventh decades were more likely to be diagnosed with MM. The median age of diagnosis reported in this study is 62 years, comparable to the African American population (65 years) but lower than the Caucasian population (71 years). Early-onset MM is more common in Sub-Saharan Africans, contrasting with this study. Most studies reported in other sub-Saharan African countries had onset of MM ranging from 53 to 62 years [15-36].
Regardless of race, men are significantly more likely than women to be diagnosed with MM. However, research from Cameroon and Sudan found considerable variances in male-female distribution. In our study, there were more males than females [32,36,37].
The inability to make an early diagnosis of MM constitutes a substantial obstacle in its management in Sub-Saharan Africa. Similar to other studies on multiple myeloma, most of our patients also presented in late-stage III and II. The prognosis is usually dismal in these advanced phases, and palliative care becomes the best option to manage such patients [8,15,32-34].
The most common presenting complaint in this study was bone pain, which affected more than 95.7 percent (178/186) of the patients with MM. This is similar to previous research and is expected as it is among the earliest and commonest clinical presentations in patients with multiple myeloma. In this study, patients were managed with different anti-pain medications depending on the severity of the pain. Drugs used included paracetamol, non-steroidal anti-inflammatory drugs such as meloxicam, opioids such as tramadol and some patients with very severe pain received morphine. According to previous research, back discomfort was also common in Burkina Faso and Ghana, which was also seen in the current study. The spinal column and skull were the most common sites for lytic lesions in our investigation, as in previous studies [2,29-32,38].
Only three patients in this study could afford the immunofixation test; this could be due to its high cost. We could not analyze as only three patients could afford this test; however, these three people had an IgA: IgG-type myeloma ratio of 1:2. This is consistent with previous research by Salawu and Durosimi, as well as Fasola, et al. Out of 117 patients, only 4 had solitary plasmacytoma confirmed histologically. This is similar to research conducted in Ghana and Nigeria, where only three out of 169 and three out of fifteen patients were diagnosed with solitary plasmacytoma, respectively [3,7,8,10,12,35,39,40].
From the earliest use of Melphalan to the introduction of stem cell transplantation and, most recently, the period of innovative targeted medicines, there have been substantial improvements in the treatment of MM. Several combinations of drugs that have proven activity in multiple myeloma have been developed. Only three (3) treatment regimens are available and are currently used to treat patients with MM in Tanzania. These include BTD/BLD, MP, and MPT. Similar regimens seem to be used in sub-Saharan Africa, Asia, and a few western countries.
In contrast to these findings, other treatment regimens are also found and widely used on top of the ones present in Tanzania in countries like Ghana, China, and the west. These include VAD (vincristine-doxorubicin-dexamethasone), CBV (cyclophosphamide combined with BCNU and etoposide), and vincristine, Adriamycin (DOX), and Dexamethasone (VAD). Many of these other treatment regimens are unavailable due to their high cost [32,35-38,40,41].
Bortezomib, thalidomide, and Melphalan are not covered by Tanzania’s National Health Insurance, so the patient’s financial situation influences their treatment regimen selection. Patients must buy these medications by themselves, even if they are insured. Age and comorbidity also affect the choice of treatment. In this study, most patients received BTD (51% (60/117)) as the treatment for myeloma, followed by MP and MPT. This is comparable to a study conducted in China by J LU, et al., and it could be because BTD was first introduced in Tanzania in 2017 with a lot of good response and hope for increased survival outcomes, as proved in most literature, while MP and MPT had been there for the past 20 years. Contrary to these findings, countries like Nigeria and Kenya with similar treatment regimens have more patients treated with MP followed by MPT and BTD [8,13,33,36].
BTD regimen showed superior response compared to MP and MPT, as most patients with BTD attained complete remission. The efficacy of BTD unlike MP, MPT and other ant myeloma drugs is due to the ability of Proteasome inhibitors (PIs) to induce the accumulation of unfolded and misfolded proteins, leading to apoptosis and cell death through ER stress, reactive oxygen species production, JNK and p53 activation, cyclin-dependent kinase inhibitors, and pro-apoptotic proteins induction. The classical mechanism of action of bortezomib on MM cells is an inhibitory effect on the transcription factor nuclear factor-κB (NF-κB) pathway activation, which is essential for myelomagenesis. These results are comparable to a study conducted by Eslam S.M. Bassiony, et al. that showed Bortezomib with Dexamethasone improved response rate and survival outcome in Egypt in 2017. In this study, therapy was stopped or changed because of toxicity or treatment failure. Most studies worldwide indicate similarities with this study and concur that the Bortezomib regimen has a better therapeutic outcome [40,42-44].
There was a slight increase in mean hemoglobin levels with all treatment regimens, a slight decrease in creatinine levels, and a reduction in serum calcium levels among all patients after treatment. This could be a result of supportive treatments given to all patients, such as analgesics (opiates and nonsteroidal anti-inflammatory drugs [NSAIDs]), hematinics, bisphosphonates (such as pamidronate, Alendronic acid, or Zoledronic acid) for hypercalcemia and bone disease, and occasionally erythropoietin (erythroid growth factor). Hemodialysis was performed on some patients who had evidence of renal damage, and aspirin was provided to all patients receiving immunomodulatory drugs unless contraindicated. Even though these results across the treatment groups are not statistically significant, similar reports have been observed in many sub-Saharan countries and other parts of the world [8,15,34,42].
Peripheral neuropathy and cytopenia that involved mostly severe anemia and thrombocytopenia were the most reported and statistically significant complications of multiple myeloma therapy in this study. This could generally be due to the disease itself or treatment-related complications. However, most myeloma-related complications improve with disease response to therapy. These results were considered in patients who did not have these complications before treatment but developed them after that and those who had presented with the progression of these symptoms after starting treatment. Therefore, pre and post-treatment neuropathy was differentiated by considering patients who did not have neuropathy before treatment but developed them after that and those who presented with worsening of neuropathy after starting treatment. Bortezomib and thalidomide therapy are more likely to be associated with neuropathy. Thrombocytopenia and mild neutropenia are rare side effects of bortezomib and thalidomide. These results are similar to other studies done in Italy, Belgium, and Greece. Both thalidomide- and bortezomib-induced neuropathies are cumulative and dose-dependent. However, there is substantial evidence of clinical improvement in bortezomib-induced neuropathy following termination than thalidomide-induced neuropathy [12,45-50].
Deaths were roughly 50% (39/77) in those who had the MP regimen since the majority of patients in this group had progressing disease. The lowest death rate was recorded in those who received MPT, i.e., approximately 20% (2/9) of participants. Despite the reduction in early mortality shown in patients treated with novel medicines, significant toxicities continue to be associated with an increased risk of death. Melphalan-based regimens are also strongly linked to disease progression and an increased risk of death [51].
This is the first study in Tanzania that has assessed complications and response to treatment regimens in patients with multiple myeloma, so it stands as a baseline for further future studies. This study is limited by presence of a lot of missing data and a lot of patients who were lost to follow up. Also, all participants were not evaluated for genetic abnormalities and only few patients had serum immunoglobulins analyzed due to the high cost of this test. Lack of enough facilities to do the ß2 microglobulin test made it impossible to stage the patients using the New International Prognostic Staging System.
Most patients were diagnosed with MM at a median age of 62 years, and the majority presented with stage III disease. A lot of patients received BTD as the treatment for myeloma, followed by MP and MPT. BTD regimen showed superior response compared to MP and MPT, as most patients with BTD attained complete remission. Peripheral neuropathy and cytopenia were the most common and statistically significant reported complications.
• Additional research, preferably prospective research.
• The national health insurance to cover at least half if not all the cost of chemotherapy for multiple myeloma so that more patients receive BTD as most enrolled patients were insured.
• To profit from recent breakthroughs in the management of MM, the government must provide equipments for assessing ß2 microglobulin (for the International Prognostication Staging System), interleukin-6, C-reactive proteins, immunofixation, immunoglobin quantification, and immunophenotyping.
• With BTD, there is room for pursuing transplant which gives high survival rates. Tanzania began performing ASCT at a lower cost than the rest of the world in late 2021. Therefore, the government should choose to financially support the BTD regimen.
Approval to conduct this study was sought from Research and Publications Committee of MUHAS and permission to conduct this study was obtained from MNH authorities. During and after the study, confidentiality was maintained by safely keeping the study materials. De-identification at data entry was done to minimize the direct association of patients’ details with the data. Only personnel directly involved with the research had access to the data. Information gathered will be used only for purposes of study and resultant publication. Verbal consent was used for relatives contacted over the phone to provide information on patients lost to follow-up and those who had either missing or discrepancies in their records.
The authors have no competing interests.
This research was funded by the Tanzanian Ministry of Health, Community, Development, Gender, Elderly and Children. These funders were not involved in study designing, data collection, analysis or report writing.
DK conceived and designed the study. She also collected and analyzed the data, and wrote the paper; CC participated in study designing, proposal development, analysis and preparation of manuscript; NB participated in study designing, proposal development, analysis and manuscript preparation; SM participated in study designing, proposal development, analysis and manuscript preparation.
Special thanks goes to my supervisors and staffs at MUHAS and MNH (departments internal medicine (HBT unit) and medical records) for their valuable support during all stages of this study. It is through their hard work, commitment and dedication to furtherance hematology research that we are able to present this paper. My sincere gratitude to the Ministry of Health, Community Development, Gender, Elderly, and Children (MoHCDEC) of Tanzania for funding this study.