Corticotropin releasing factor (CRF) is involved in conditions of anxiety and stress: It stimulates the release of adrenocorticotropin hormone (ACTH). Also, catecholamines increase ACTH release, while serotonin (5-HT) increases both ACTH and CRF.
The electrochemical technique of voltammetry used in association with specifically treated carbon fiber microelectrodes (µCFE) allows the in vivo detection of catecholamines, serotonin and peptides in discrete brain regions, simultaneously. This methodology can therefore be combined with behavioral models of stress-anxiety-depression.
Preliminary data are presented as well as experimental proposals so that to analyze the feasibility to set an experimental behavioral-neurochemical in vivo model of stress-anxiety-depression. In particular, to verify a direct relationship between 5-HT and ACTH systems in discrete brain areas highly involved in several physiological and behavioral activities such as emotion, vigilance, memory, and adaptive responses to stress with the aim to the possible formulation of new strategies of pharmacological treatment(s) of such pathological state(s).
Corticotropin releasing factor (CRF), Stress-anxiety-depression, In vivo voltammetry, Catecholamines, Serotonin (5-HT), FST test
Corticotropin releasing factor (CRF) and arginine vasopressin (AVP), both of which are synthesized in the hypothalamus, stimulate the release of adrenocorticotropin hormone (ACTH) from the anterior hypophysis in conditions of stress, anxiety [1-4].
Moreover, in rodents, central catecholamines stimulate ACTH release, effect mediated via secretion of CRH (for a review see [5], while serotonin (5-HT) increases both ACTH and CRF release and facilitates the capacity of AVP to release ACTH in stressful situations [6-9].
Voltammetry and in particular differential pulse voltammetry (DPV) is an electrochemical technique that used in association with specifically treated carbon fiber microelectrodes (µCFE) allows the detection of catecholamines, serotonin and peptides simultaneously in discrete brain regions of anaesthetized as well as conscious freely moving rats [10,11].
This methodology can therefore be combined with behavioral models of stress-anxiety-depression such as forced immobilization that mingles emotive and physical stress [12] and for a review see [13], or forced swimming test [14,15] or fear conditioning [16,17].
This methodological combination will allow monitoring selective changes of the monoamine neurotransmitters in real time and in the specific cerebral region selected within animals submitted to such aversive conditions. In particular, limbic system components such as amygdala, hypothalamus and regions reciprocally connected with the amygdala such as the hippocampus, are involved in emotional responses to aversive stimuli [18-20]. Therefore these regions could be studied. In particular, amygdala could be the prior target as it has been reported that 25% of the neurons of this area contains CRF [21,22].
Additionally, it is reported that the locus coeruleus (LC) has been involved in several physiological and behavioral activities such as emotion, vigilance, memory, and adaptive responses to stress [23]. Stressful stimuli trigger LC neurons, modify their electrophysiological activity, and induce release of catecholamines [24]. LC contains the largest amount of catecholaminergic cells in the brain and innervates large sections of the neuroaxis [25,26]. Its stimulation determines ACTH release and anxiogenic-like behaviors among other stress-associated responses (for a review see [27]). Interactions between CRF and central catecholamines has been already reported as well as that administration of CRF in CNS alters activity of LC neurons and catecholaminergic metabolism in terminal areas and stimulates catecholamine release in hypothalamus and prefrontal cortex (for a review see [27]). Such an altered function of LC neurons has been implicated in the pathophysiology of affective and stress-related disorders [4,28].
Extensive rodent and human research has shown that the hippocampus is highly sensitive to stress and that prolonged exposure to stress leads to loss of neurons, particularly in the hippocampus [29]. The hippocampus also plays an important role in the terminating HPA axis responses to stress [30]. Hippocampal lesions increase parvocellular CRF and AVP expression, prolong ACTH and corticosterone release in response to stress [31] and the hippocampal outflow to the hypothalamus originates in the ventricle subiculum and CA1 regions of the hippocampus (CA1) i.e. terminal region of LC [30]. Furthermore, involvement of the 5-HT in mediating stress effects on the hippocampus is proposed by data showing that stress elevates 5-HT levels in the hippocampus [32].
Based on the previous studies indicating the locus coeruleus and the hippocampus as among the most interested brain regions by stressful situations, preliminary studies have been performed in these two structures. The electrochemical technique of voltammetry used in association with specifically treated carbon fiber microelectrodes (µCFE) has been applied for the in vivo detection of catecholaminergic and serotoninergic levels, simultaneously. Concomitant cell firing measurements have been performed in the LC. This association of electrochemical and electrophysiological techniques can be combined with behavioral models of stress-anxiety-depression [33].
Preliminary data are presented as well as experimental proposals so that to analyze the feasibility to set an experimental behavioral-neurochemical in vivo model of stress-anxiety-depression. In particular, to verify a direct relationship between 5-HT and ACTH systems: With the aim to the possible formulation of new strategies of pharmacological treatment(s) of such pathological state(s).
Differential Pulse Voltammetric (DPV) and electrophysiology were applied in vivo by means of µCFE prepared as described earlier using carbon fiber with diameter 30 µm. They were first electrically treated, i.e. a 70 Hz triangular wave form was applied in three stages: 0 to +2.2 V for 8 s, 0 to +1.8 V for 10 s, and 0 to +1.2 V for 10 s. Two successive, continuous potentials were then applied: -1.0 and +0.5 V, for 4 s each. This electrochemical treatment was carried out with the auxiliary, reference, and working electrodes immersed in 0.1 M Phosphate-Buffered Saline (PBS) at pH 7.4. Then the tip of the electrodes was coated with Nafion so that selective, simultaneous measurements of catechols (noradrenaline, dopamine) and serotonin (5-HT) could be performed [34,35].
Male adult rats (Wistars, 250-280 g) were supplied by Charles-River (Italy) and kept in temperature- and humidity-controlled rooms (22 ℃, 50%). All animal procedures were carried out in accordance with the Italian law (Legislative Decree no. 116, 1992) which acknowledges the European Directive 86/609/EEC and were fully compliant with the GlaxoSmithKline policy on the care and use of laboratory animals and codes of practice. Furthermore, all efforts were made to minimize the number of animals and their suffering.
The animals were anaesthetized (chloral hydrate, 500 mg/kg i.p.), set in a stereotaxic frame and prepared for concomitant differential pulse voltammetric (DPV) and electrophisiologic analysis as described earlier [33,35].
In particular, one DPV micro-biosensor was stereotactically inserted in the locus coeruleus (LC) i.e. cell body region, then, in the same animal, a second µCFE was implanted within the hippocampus (CA1) i.e. terminal region of LC, following coordinates from Paxinos and Watson [36] (See Figure 1).
 Figure 1: Scheme of coupled voltammetric (potentiostat/galvanostat) and electrophysiological (electrophysiologic unit) instruments for concomitant DPV monitoring of catechols levels and electrophysiological measurements of cell firing.
Figure 1: Scheme of coupled voltammetric (potentiostat/galvanostat) and electrophysiological (electrophysiologic unit) instruments for concomitant DPV monitoring of catechols levels and electrophysiological measurements of cell firing.
HIPP: Hippocampus; LC: Locus coeruleus; A: Auxiliary; R: Reference; microCFE: The three electrodes for DPV scans i.e. in hippocampus; microCFE in LC and G: Ground: The two electrodes for electrophysiological measurements i.e. in LC.
Electrodes A, R and G are made with silver wire (see Crespi, et al. [34]).
View Figure 1
Both microsensors were previously chemically treated with Nafion so that selective, simultaneous DPV measurements of catechols (noradrenaline, dopamine) and serotonin (5-HT) could be performed [11,33,35].
Successively, in vivo differential pulse voltammetric (DPV) measures of neurotransmitters as well as electrophysiologic detection of cell firing have been performed as described earlier [11,33,35].
In these animals, intracerebroventricular (i.c.v.) infusion of CRF (1 µg) or aCSF (4 µl artificial cerebral spinal fluid: Control rats) determined the results shown in Figure 2, Figure 3 and Figure 4. In particular, these data show that CRF significantly increases cell firing in LC (Figure 2) as well as catechol levels in CA1 (Figure 3). In contrast, no significant changes have been observed in 5-HT levels (Figure 4).
 Figure 2: Electrophysiology (cell firing) in LC of rats treated (arrow) with CRF (■ 1 µg/4 µl, n = 6) or with aCSF 4 µl (□ control rats, n = 6) Data are expressed in % of control, **p < 0.001, *p < 0.05.
Figure 2: Electrophysiology (cell firing) in LC of rats treated (arrow) with CRF (■ 1 µg/4 µl, n = 6) or with aCSF 4 µl (□ control rats, n = 6) Data are expressed in % of control, **p < 0.001, *p < 0.05.
Stats: Effect of group: F(1,6) = 20.1, p = 0.004; Effect of time: F(12,7) = 11.9, p = 0.001; Group by time interaction: F(12,7) = 14.1, p = 0.001.
View Figure 2
 Figure 3: DPV levels of catechols in hippocampus CA1 of rats treated (arrow) with CRF (■ 1 µg/4 µl, n = 6) or with aCSF 4 µl (□ control rats, n = 6) Data are expressed in % of control *p < 0.05.
Figure 3: DPV levels of catechols in hippocampus CA1 of rats treated (arrow) with CRF (■ 1 µg/4 µl, n = 6) or with aCSF 4 µl (□ control rats, n = 6) Data are expressed in % of control *p < 0.05.
Stats: Effect on time F(4,2) = 6.1, p = 0.002; Effect on group F(1,6) = 4.459, p = 0.05 and group by time interaction F(4,2) = 5.6, p = 0.002.
View Figure 3
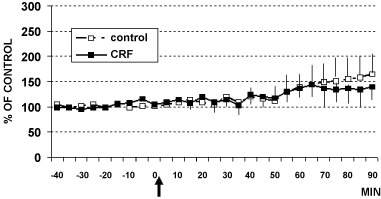 Figure 4: DPV levels of serotonin (5-HT) in hippocampus CA1 of rats treated (arrow) with CRF (■ 1 µg/4 µl, n = 6) or with aCSF 4 µl (□ control rats, n = 5) Data are expressed in % of control.
View Figure 4
Figure 4: DPV levels of serotonin (5-HT) in hippocampus CA1 of rats treated (arrow) with CRF (■ 1 µg/4 µl, n = 6) or with aCSF 4 µl (□ control rats, n = 5) Data are expressed in % of control.
View Figure 4
The present data on modification of catechol activity following such treatment are in accord with reports indicating a direct effect of intracerebroventricularly infused catecholamines, acting via alpha1 and/or beta-receptors, on induction of stress-like ACTH surges in rats [27,37]. The preliminary DPV data obtained in LC indicated again changes in catechol levels but no significant changes in 5-HT levels (Figure 5 and Figure 6). In contrast, other work has shown a role of LC and serotonin in mediating the effect of exposure to stressors [38]. The discrepancy could be related to the different methodology applied, i.e. push pull canulae, with possible blood contamination of the superfusate collected, however, further studies, as proposed hereafter will help to elucidate that divergence.
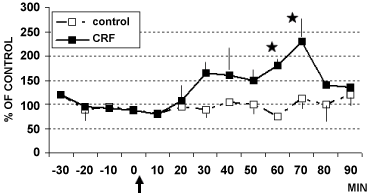 Figure 5: DPV levels of catechols in LC of rats treated (arrow) with CRF (■ 1 µg/4 µl, n = 4) or with aCSF 4 µl (□ control rats, n = 6) Data are expressed in % of control *p < 0.05.
Figure 5: DPV levels of catechols in LC of rats treated (arrow) with CRF (■ 1 µg/4 µl, n = 4) or with aCSF 4 µl (□ control rats, n = 6) Data are expressed in % of control *p < 0.05.
Stats: Effect of group: F(1,20) = 4.6, p = 0.004; Effect on time: F(5,1) = 16.8, p = 0.0001; Group by time interaction: F(5,1) = 9.2, p = 0.0001).
View Figure 5
 Figure 6: DPV levels of serotonin in LC of rats treated with CRF (■ 1 µg/4 µl, n = 4) or with 4 µl aCSF (□ control rats, n = 6) Data are expressed in % of control.
View Figure 6
Figure 6: DPV levels of serotonin in LC of rats treated with CRF (■ 1 µg/4 µl, n = 4) or with 4 µl aCSF (□ control rats, n = 6) Data are expressed in % of control.
View Figure 6
Row data were subjected to ANOVA, with comparison between "control" (vehicle) and "treatment" values performed using the Bonferroni (Dunn's) test. Then, the results were presented as % of control values, mean ± s.e.m., *p < 0.05, **p < 0.001.
Various groups of rats (n = 5 each group) can be prepared for concomitant voltammetric measurements and electrophysiological recordings and then exposed to forced swimming test (FST) as already described [33] in order to monitor the influence of such stressful conditions upon monoaminergic activities.
FST is a behavioral test that predicts the clinical efficacy of many types of antidepressant drugs [14].
By means of the same microbiosensor (µCFE) used for voltammetric measurements electrophysiological recordings can be also performed [33]. Thus, parallel double probing analysis of amine neurotransmitter activities and firing levels could be monitored in the amygdala of naïve control (n = 5) versus "FST behaviorally treated" rats (n = 5). Similar studies could be performed in the Raphe Dorsalis Nucleus (RDN), the richest brain region of serotonin cells and presenting clear interconnections with the amygdala [39,40]. This further study performed in five control rats versus 5 "FST rats" would allow to specifically analyzing the serotonergic system at the level of cell bodies (RDN) as well as at the level of synaptic cleft (amygdala). The reduced activity of such system in patients with unipolar depression is indeed well known [41,42].
In further four groups of rats prepared as above for parallel double probing analysis of amine neurotransmitter activities and firing levels in the amygdala and in RDN and submitted to FST the following treatments could be performed:
i) Vehicle (aCSF 4 µl, control rats, n = 5), ii) CRF (1 µg, n = 5), or iii) AVP (1 µg, n = 5), or iiii) ACTH (1 µg, n = 5), (or isoproterenol: Compound that releases ACTH [43]).
The data gathered from these two set of experiments tests will allow analyzing:
a) The influence of a behavioral test predictive of the clinical efficacy of many types of antidepressant drugs upon monoaminergic systems in discrete brain areas highly implicated in several physiological and behavioral activities such as emotion, vigilance, memory, and adaptive responses to stress.
b) The influence of the selective chemical treatments with compounds involved in the response to stressful stimuli upon monoaminergic activities in rats submitted to stress-anxiety-depression.
These studies will permit to verify the setting up of an experimental behavioural-neurochemical in vivo model of stress-anxiety-depression i.e. the concomitance of the electrochemical and electrophysiological outcome following behavioral or pharmacological treatments in naïve versus rats submitted to stress-anxiety-depression would clarify the role of CRF (and ACTH) as the putative endogenous responsible of a stress-anxiety-depressive state.
This experimental behavioral-neurochemical in vivo model will permit to verify the efficacy of CRF antagonists upon behavioral responses and electrochemical-electrophysiological parameters, data that will underline the efficacy of such compounds within the stress-anxiety-depressive state. Briefly, rats submitted to behavioral model(s) of stress-anxiety-depression or/and rats treated with CRF (isoproterenol, ACTH) will be previously treated with CRF antagonists. Then concomitant behavioral and neurochemical activities will be monitored in real time.
Furthermore, the following (among other) additional evidence from literature are underlying the direct relationship between 5-HT and ACTH systems:
• The 5-HT1A receptor agonist 5-OH-DPAT as well as the 5-HT2 receptor agonist DOB increase plasma ACTH (and corticosterone). DOB is probably acting via CRF as pretreatment with CRF antagonist (alpha-helical CRF9-41) significantly attenuates the ACTH response to DOB [44-46].
• 5-HT depleters (i.e. 5,7-DHT, PCPA) while reducing 5-HT levels, also result in decreased levels of ACTH [47-49].
The validity of animal's models of human mental disorders is generally evaluated by a set of conditions and chronic stress is among those showing the main validity in rats (for a review see [50]). In particular FST appears to be a useful approach to study in rodents (positive) influence of drugs on clinical treatment of major depression [51].
Thus, the proposed experimental behavioural-neurochemical in vivo model in combination of pharmacological treatments aiming to these two systems (i.e. association of CRF receptor antagonists and 5-HT receptor antagonists) could be useful in the understanding of their concomitant implication(s) within behavioral models of stress-anxiety-depression states and therefore possible formulation of new strategies of pharmacological treatment(s) of such pathological state(s).