Community-acquired cases of Legionella infection or even outbreaks can be attributed to inhalation of aerosols from devices such as hot water system, cooling towers, hot tubs, industrial equipment and indoor fountains. Legionellae survive in water in temperatures between 20 ℃ and 50 ℃ and tend to colonize particularly water systems rich of sludge, rust, biofilms and amoebae where they can multiply. Cooling towers (CT) in industry are used as heat-transfer devices in which warm water is cooled by evaporation in atmospheric air. Aerosols can transmit Legionellae to susceptible hosts, who can contract either Legionnaires´ disease (elderly with many risk factors) or Pontiac fever (young or middle-aged people relatively healthy without any risk factors).
Aim of the study was risk assessment of legionellosis for workers in contact with contaminated water aerosol from industrial cooling towers.
Water samples from industrial cooling towers and air samples were processed by standard manner (EN ISO 11731) and plated on special buffered charcoal yeast extract agar with 0.1% ketoglutarate and L-cysteine (BCYα medium) containing glycine, vancomycin, polymyxin B, cycloheximide (GVPC medium) for Legionella isolation. Exposure of workers to water aerosols was evaluated by interview, questionnaire, serological testing (agglutination test), cultivation of sputum on BMPA (BCYα medium with cefamandole, polymyxin B, anisomycin), detection of Legionella antigen in urine by ELISA and DNA Legionella in sera by PCR.
Sampling water from 6 cooling towers revealed isolates of Legionella pneumophila (L.p.) serogroups 1, 5, 10 from four of them (1,6 × 102 - 1,49 × 104 /200 ml). Investigation of air around three CT showed contamination by L. p. serogroup 12 in one of them. Antibodies only against this L. p. serogroup 12 were detected in single sera (1:128 - 1:256) in 13 workers, i.e. in two external workers working directly inside CT (diving) and 11 internal workers, who attended instruction meeting lasting several hours close to this tower. The workers contracted non-pneumonic infection - Pontiac fever with mild clinical symptoms.
Exposure to water aerosols produced by the industrial cooling tower led to the cluster of non-pneumonic professional Legionella infection in workers. Results of the study were used for recommendation of repressive (disinfection, operating regimen of towers) and preventive (respiratory protective equipment, monitoring of Legionella colonization, etc.) measures.
Legionella, Aerosol, Industrial cooling tower, Risk assessment, Control measures
Legionella is a widespread bacterium usually in low numbers present in natural environmental water sources such as rivers, lakes and from them it passes into sites that constitute artificial reservoirs (channeled water in towns, hotels and hospitals, cooling towers in buildings and industry, etc.). Temperatures in the range of 20 ℃ to 45 ℃ together with stagnancy, sediment, sludge, scale, rust and microbial multispecies biofilms with protozoa within the water system provide favorable conditions for Legionella growing [1]. Free-living thermo-tolerant amoebae play a crucial role in the lifecycle of Legionella species as they provide a habitat for Legionella environmental survival and replication and also this intra-cellular niche serves for protection from environmental stressors including biocides and heat treatment of water system. The growth of Legionella in biofilms may lead to enhanced virulence [2].
Although more than 60 different species of genus Legionella have been described, around 28 species have been associated as opportunistic pathogens with human disease, of which Legionella pneumophila (L. p. 1-15 serogroups) accounts for almost 80-90% of the diagnosed clinical cases [3-5]. Up to 90% of reported cases of legionellosis in the US and Europe are caused by Legionella pneumophila serogroup 1 (Lp1), but also colonization of water by other L. p. serogroups and species could pose risk of legionellosis [3,6].
Two forms of legionellosis could be acquired by inhalation Legionella contaminated water aerosol or by microaspiration in hospitalized patients with severe health conditions [3,4,6,7]. Legionnaires' disease (LD), occurring after an incubation period usually 2 to 10 days, is a serious form of pneumonia in sporadic or epidemic intensity with a case-fatality ratio 10-15% with shock, respiratory and multi-organ failures. LD predominates in males (ratio 2:1) with risk factors (older than 50 years, smokers, severe chronic diseases, transplantation, immunodeficiency, etc.) [7,8]. Legionella infections in Europe are classified as nosocomial (10%), travel-associated (21%), and community-acquired (69%), similar as in other countries with precise surveillance [5]. The other non-pneumonic form of Legionella infection known as Pontiac fever (PF) is an influenza-like, self-limiting illness with shorter incubation period, normally 12-48 hours, lasting few days. The attack rate of PF is much higher than for Legionnaires' disease (up to 95% of those exposed) and cases are mainly detected when outbreaks of Pontiac fever occur [9,10].
Diagnostics of both forms is based on detection of Legionella antigen in urine, antibodies in sera, PCR and culture on specific selective media [3,11,12].
Only Legionnaires' disease not Pontiac fever is a statutorily notifiable disease in all EU/EEA Member States. Rates of disease vary from 1.0 to 30.0 cases per million population. In 2017 thirty countries (27 European Union Member States and 3 EEA states) reported 9, 238 LD cases (incidence 1.8 per 100,000) with 8% case fatality rate. Legionnaires' disease remains an uncommon and mainly sporadic respiratory infection with a continuously increasing annual overall notification in Europe [5]. For comparison, 4,202 cases across the United States (incidence rate of 1.36 cases per 100,000) were reported in 2013 with rising incidence and in Australia recorded 2.2 cases per 100,000 in 2014. The true incidence of legionellosis may be much higher as many cases are unreported [5,13].
In general, every Legionella contaminated water device producing aerosol can carry the risk for Legionella transmission and cause either LD or Pontiac fever [6,10,14]. Reported legionellosis outbreaks with both LD and PF are relatively rare [10]. Virulence and viability of Legionellae, size of respirable particles and susceptibility of exposed people are decisive for disease development.
Evaporative cooling of water with cooling towers and evaporative condensers are widely used to dissipate heat from air conditioning, refrigeration and industrial process systems. They include mostly open-circuit cooling towers that can range in size from small towers used in air conditioning to large towers for heavy industrial applications [15]. Aerosols generated from open cooling towers can through water droplets transmit legionellae over large distances to susceptible hosts [16] and cause legionellosis in community [10,14] as well as in hospitals [17]. It is imperative that CT are managed to prevent legionella colonization and the potential release of contaminated aerosols. Therefore disinfection by biocides, control of quality of recirculating water in storage tanks/ponds of CT and monitoring of legionellae, biological and chemical conditions favorable for legionellae are most important preventive measures [1, 15,18]. Control and registration of cooling towers can prevent some legionella community acquired and nosocomial outbreaks, of special concern are industrial CT [1,14,19,20].
AIM of the study was risk assessment of professional exposure to water aerosols for persons operating or working close to cooling towers (CT) in a petrochemical factory who complained of respiratory discomfort.
An investigation - retrospective study was carried out to determine the risk to acquire legionella infection in professional activity. Assessment of hazards involved collecting and investigation of water samples from CTs, air sampling, biological samples of employees and evaluation of information of any clinical symptoms of the workers and exposure to water aerosol.
CT was performed by processing water samples from pools and recirculating water according to EN ISO 11731 standard [21]. 500 ml water samples were collected in sterile bottles and analyzed on the same day. Water volume of 200 ml was concentrated by membrane filtration (0.22 um), then the membrane was cut and its microbial content was diluted by shaking in 10 ml sterile water for 5 min. This water sample was divided into three portions before culture. The first portion (0.5 ml) was directly plated, the second one (3 ml) was processed by heat treatment (50 ℃/30 min) and the third portion (3 ml) was acid pretreated by 0.2 M HCL- KCl (pH 2.2) 1:1 during 5 min. All portions in volumes of 0.5 ml were plated onto buffered charcoal yeast extract agar with 0.1% ketoglutarate and L-cysteine (BCYE α medium) containing glycine (3 g/L), vancomycin (1 mg/L), polymyxin B (80,000 UI/L) and cycloheximide (80 mg/L) - GVPC medium and incubated at 35 ℃ in a 2.5% CO2 atmosphere 10 days. Morphologically Legionella suspected colonies were plated on blood agar (BA) and those which failed to grow on BA were cultured on BCYEα. Typing of isolated Legionella colonies was done by latex agglutination test (Oxoid) and by agglutination test using polyclonal rabbit immune sera against 15 serogroups of L. pneumophila (L. p.) and 5 Legionella like organisms (LLO) - L. bozemanii, L. micdadei, L. dumoffii, L. gormanii and L. longbeachae.
Air sampling was performed in summer time (no windy weather) by portable impactor SAMPL'AIR MK2® (BioMérieux) in volume of 100 L during 1 min. (according to calibration of the air sampler) in respirable zone (1.6 m high above the ground, 1.5 m distance from the towers). The air was aspirated directly onto GVPC medium and each sampling was done in duplicate. The GVPC media were immediately stored under cool conditions 2 hours and then incubated as usually. Air sampling was performed by standard procedures for testing and evaluation of air pollution used in our country [22] and in accordance with international standard EN ISO/IEC 17 025:2005 and protocol for Legionella testing EN ISO 11731 [21]. The head of the air sampler was sterilized by autoclaving (121 ℃/20 min.). Interval between samplings was 10 - 30 min. with disinfection of the air sampler by ethanol. Legionella contamination of air samples was calculated as the mean of colony counts on both duplicate plates times factor for calibrated volume of aspirated air. Finally, total colony count was expressed as CFU/m3.
We defined a case of Legionella infection as an employee/worker with potential exposure to water aerosol from CTs with or without flu like symptoms (fever, headache, myalgia, nonproductive cough) with onset since mid - May who was positive of Legionella infection by culture, Legionella urinary antigen test (UAT) or testing antibodies to L. pneumophila or LLO in sera (titer ≥ 1:128). Two or more cases exposed to the same site in the range of 1 to 10 days before onset of illness was classified as cluster.
Questions in interview concerned mainly profession characteristics - categorization of the work targeted to exposure to water aerosol at work vs. in free time, subjective complaints (respiratory symptoms and others) recently or in previous time (10 days before the study), risk factors (smoking, other serious diseases) and wearing personal protective tools (respirator, mask) and demographic data (age).
Serological testing by agglutination reaction (AR) with 21 heat treated legionella antigens (L.p. serogroup 1-15, including two strains of L.p.1 - Philadelphia, Knoxville, 5 LLO) with cut off titer 1:128.
Sputum cultivation on selective BCYEα with cefamandole (4 mg/L), polymyxin B (80,000 UI/L) and anisomycin (80 mg/L) - BMPA medium for legionella growing. The sample according to density was diluted 1:1 with 0.1% dithiothreitol and incubated 15 min. at 35 ℃. Then the sample underwent with an equal volume acid treatment by 0.2 M HCL- KCl buffer (pH 2.2) for 5 min. and 0.1 ml of the sample was plated onto BMPA agar and incubated for 10 days at 35 ℃.
It was performed by commercial ELISA test (Biotest, Germany) for detection L. pneumophila and some LLO (unknown spp.) performed by instructions of the manufacturer. Urine samples were pretreated by boiling for 5 min and concentrated by centrifugation (4,000/10 min) using supernatant for testing.
Detection of DNA legionella in sera by PCR (Phoresis, Russia) targeted to L. pneumohila gene 16S rRNA and in -house PCR with the primer to gene mip (macrophage infectivity potentiator).
Statistical testing was performed by Student t-test, p ˂ 0.001 was evaluated as significant difference between two values.
Water sampling from six cooling towers (on 2nd June, on 27th June) detected legionellae in five of them (Table 1) in concentrations 1.6 × 102 - 1.49 × 104/200 ml. Temperature of an output water was 15 - 23 ℃ while input recirculated water was warmer 32 - 36 ℃.
Table 1: Sampling water from cooling towers (CFU/200 ml). View Table 1
Legionellae isolates in latex agglutination tests and in agglutination reactions with polyvalent hyperimmune rabbit sera were identified as strains of Legionella pneumophila species serogroup 1 (non - Pontiac subgroup - OLDA), L.p. serogroup 5 and L.p. 10.
Sampling air around three towers (on 31st July) revealed legionellae only in samples from the tower No. 4 (15 CFU/ m3) which were identified as L. pneumophila serogroup 12 and L. bozemanii serogroup 1 (Figure 1).
 Figure 1: Sampling water, air and sera of workers - time chart. View Figure 1
Figure 1: Sampling water, air and sera of workers - time chart. View Figure 1
Questionnaires with informed consents were filled by 23 male workers (17 internal, 6 external) in mean age of 42.8 years (range 24 - 57). External workers (mean age 33.5 years) worked on construction and mending CTs were exposed to water/water aerosol during 45 and 15 hours per week. Internal workers (mean age 46.1 years) worked mainly as operators exposed to water from CTs 2 - 12 hours/week (Figure 2).
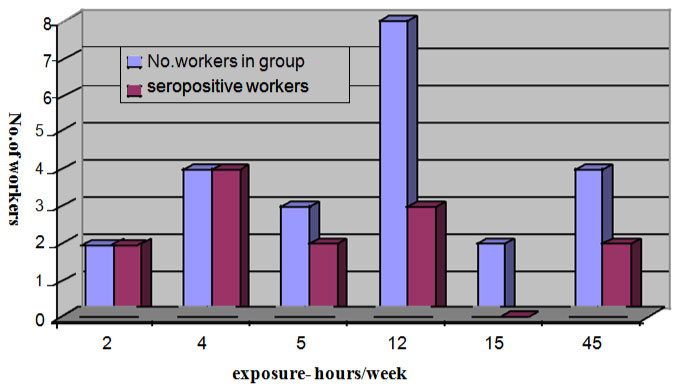 Figure 2: Seropositivity of workers in groups with different exposure time to water from cooling towers. View Figure 2
Figure 2: Seropositivity of workers in groups with different exposure time to water from cooling towers. View Figure 2
Epidemiologically important for analysis was the participation of 14 internal workers at the instruction meeting close to the 4th CT which was held on 19th June - 22nd June (Figure 1). Serological testing of single sera from 23 workers collected during 30th June - 7th July revealed antibodies to L. pneumophila serogroup (sg) 1 and L. p. 5 in low titers but against L. pneumophila serogroup 12 in diagnostic titers 1: 128 - 1: 256 in 13 workers (11 internal, 2 external workers) with no response to L. bozemanii.
Time chart (Figure 1) shows results of water and air investigations of CTs and serological testing of workers during 2nd June to 31st July. In the beginning of the survey two water samplings from six CTs were done with positive isolation of Legionellae L.p.1, 5, 10 in five of them. At the end of this study air sampling around three CTs was performed with isolation of Legionellae L.p.12 from the 4th CT. This CT was the place where the meeting was held (19th - 22nd June) and some workers took part in it. Investigations of biological samples were done during 30th June -7th July resulting in serological positivity against L.p.12 in 13 persons.
Sputum and urine collected in the same time as sera were negative in all 23 persons. Detection of DNA L. pneumophila in sera was positive in one third of the workers (8 men).
Workers were divided into groups according to exposure time (hours per week) to water/aerosol from towers. Out of 6 external workers exposed to aerosol from CTs during 45 and 15 hours per week only two (33.3%) had antibodies against L.p.12 (Figure 2). Eleven persons (64.7%) out of 17 internal workers exposed to water from CTs during 2 -12 hours/week revealed antibodies against L.p.12. Serological reactivity and duration of exposure did not show any correlation, all 6 persons with the shortest exposure time (2 - 4 hours/week) to water from CTs showed antibodies to L.p.12.
Information from questionnaires were used for comparison between groups of workers (external and internal), seropositive and seronegative with the aim to find out an association between serological positivity and exposure to water/aerosol during working activity or some other risk factors (Table 2). There was no significant difference in other free activities associated with possible exposure to water (swimming, gardening) between all workers with antibodies (38.5%) and seronegative workers (40.0%). Individual health complaints in the time of testing and in the previous time did not differ in those seropositive (30.8%, 23.1%, p - 0.69) and all workers (34.8%, 21.7%, p - 0.13), with a little bit higher rates of recent complaints in seronegative workers (40.0%) but without any statistically significant differences.
Table 2: Comparisons of all workers according to serological positivity, exposure to water in free time and subjective complaints. View Table 2
Similarly, there were no statistically significant differences of exposure to other water sources in internal workers (Table 3), between seropositive and seronegative workers (45% vs. 50%, p -0.072) or to air-condition exposure in cabin (91% vs. 100%, p - 0.32), even not among those with exposure to CTs producing antibodies and those with no serological response but attending meeting (100.0% vs. 50.0%, p - 0.016).
Table 3: Comparisons of internal workers according to exposure to water in free time, to air-condition in cabin and aerosol from CT during instruction meeting. View Table 3
It can be assumed that probably serological positivity in 11 internal workers out of 14 attending the instruction meeting close to the 4th CT originated from exposure to water aerosol from this CT with positive Legionella isolates of L.p.12. Sera were collected 11-15 days later. The difference in serological positivity was significantly higher in attendants of the meeting opposite workers with water exposure during free time (100.0% vs. 45.5%, p - 0.0003, p ˂ 0.001).
Serological reactivity of two external workers was associated with the work directly inside this CT (diving), while the others were involved in operating control activities.
Legionellosis is attributed to aerosol inhalation from devices such as hot water systems, cooling towers, hot tubs, industrial equipment and indoor fountains [1]. The largest outbreaks being caused by cooling towers [6,14]. Cooling towers in industry are used as heat-transfer devices in which warm water is cooled by evaporation in atmospheric air. Air movement through the tower or condenser is produced by fans or, occasionally, by natural convection. Aerosols can transmit legionellae to susceptible hosts and they can be infected up one mile (1.6 km) from the tower [16], mainly within 0.25 miles (400 m) of the CT [17]. Prolonged exposure (over 100 minutes) and windy weather can transmit the aerosol up to 6 km [17,23,24]. Contracting Legionella infection depends on many factors [13]. Variation in the size of aerosols affects the infectivity, which makes it difficult to determine the infectious dose and what environmental concentrations are considered acceptable, could be as little as 0.02 CFU/l [25]. Virulence of Legionellae is decisive factor, virulent strains survive in aerosol longer than avirulent ones [13]. The presence of viable but not culturable (VBNC) Legionella forms and surviving in amoebae contribute to underestimation of the real number of this pathogen detected by standard cultivation [2]. In outbreak investigation could be used PCR/r PCR and flow cytometric assay to rapidly screen potential sources [14,26], but several studies showed poor correlation with culture, giving higher contamination than cultivation yield [1]. Infection associated with frequent and extended exposure to the source, suggesting cumulative exposure could be also a risk factor for illness [17].
In our study association between contamination of aerosol from the 4th CT by Legionella pneumophila sg 12, detected by air sampling, and the same serological reactivity of workers with direct exposure to this CT during instruction meeting (internal workers) or work inside the CT (external workers) was confirmed. Legionella strain L.p.12 failed to be isolated from water or aerosols of any other CTs. Serological reactivity to isolates of L. pneumophila serogroups 1 (non-Pontiac - Olda subtype), L.p. sg 5 and L.p. sg 10 from the CT water was very low, but seroconversion in diagnostic titers only to L.p.12 was present in 13 workers. Study of prevalence of antibodies to Legionellae in healthy blood donors in Slovakia showed 4% presence of antibodies in low (1:8 - 1:32) titers to L.p. 1-15 serogroups (unpublished data), thus seroconversion in workers in this study could be the impact of professional exposure to contaminated aerosol from the 4th CT. Outbreaks, especially caused by L. pneumohila non serogroup 1 are frequently underestimated and under reported [5,26].
Pontiac fever has a high attack rate in people without any underlying disease and risk factors with recovery within one week, it preferentially affects the younger population and the median age range from outbreaks was reported to be 29-32 years, gender, and smoking have not been observed to be risk factors [9]. Workers in our study were in productive age (mean age 42.8 years). They overcame either asymptomatic or very mild infection suffering from dyspnea, caught, sore throat, diarrhea, except one (pneumonia of other etiology with quickly recovery). According to microbial diagnostic criteria [18] the cases were classified by positive serology in single sera as probable but with strong epidemiological links. The highest rate of PF was documented in 11 internal workers (64.7%) from the total number [17], but with higher rate in those who were for several hours close to the 4th CT during the instruction meeting, i.e. 11 from 14 (82.4%), three workers attending this event did not produce antibodies, what is common in about 20% of infected people [3,14]. The diagnosis of PF was not supported by urinary antigen detection (UAT) and culture of sputum, methods predominantly used in acute stage of any legionella infections. Sensitivity of UAT (Biotest) is reported to be low for detection of non-L.p.1 antigens [3,7,9]. Detection of DNA L. pneumophila in sera, which were collected later, was positive in one third of the workers.
Currently, there is no consensus as to why exposure to L. pneumophila may result in either Pontiac fever or Legionnaires disease [13,27] or occasionally simultaneous outbreaks of LD and PF from the same source have been observed [6,14,20], but many PF cases without any epidemiological analysis could be undetected [10].
Our study found that proximity of persons to contaminated water aerosol from the CT was the most important risk factor for acquiring legionellosis. In evaluation of environmental risk of cooling towers must be considered also seasonal/climatic conditions, intermittent use, poor maintenance (heavy bacterial contamination and amoebae with low disinfection) and poor design [14,20,28,29]. People living within 0.5 km of any tower were three times more likely to become infected than people living more than 1 km away [23] and the risk decreased with increasing distance [25].
The study resulted in introduction of cooling tower safety plan in cooperation with the operator of the system, which consisted of key components such as system assessment, determination of the water and aerosol quality and at the point of potential exposure, identification and monitoring of control measures used to ensure water safety (e.g. heterotrophic colony counts, biocide levels, temperature, pH) in accordance with Technical guidelines proposed by ESCIMID group (former EWGLI) in EU [1]. Control, properly treatment, regimen and maintenance with emphasis on legionellae surviving of all CTs in this industrial plant together with an emergency corrective actions had to be done. The study also underline importance of prevention of workers by wearing protective cloths, mask/respirator together with staff training and education [29].
Ten countries in Europe, have enacted legislation to register and regulate CTs, but no standard approach exists [19]. In Slovakia there is low oversight, compliance with recommendations and legionella control of potable water system, cooling towers as well as other man-made water artificial sources such as those issued by ECDC [1], WHO [20] or American National Standards Institute [28,30]. Ongoing surveillance of Legionella cases since 1985 in Slovakia [4, 5,12] and establishing the comprehensive law governing the operation and maintenance of cooling towers with the registry will also in our country facilitate identification of potential sources of any Legionella outbreak.
Small outbreaks of non-pneumonic Legionella infection (Pontiac fever) is seldom be discovered and only few epidemics were reported in industrial plants. Our study showed that environmental investigation of water/water aerosols of industrial cooling towers and the epidemiological analysis of the data from workers, i.e. laboratory data (mainly serological reactivity, culture, Legionella antigen in urine, PCR) and data about various exposures to water/aerosols (working and free time, medical history) revealed contaminated aerosol being the factor of Legionellae transmission. This professional cluster of Pontiac fever was caused by uncommon Legionella pneumophila serogroup 12. This survey confirmed that several hours of unprotected exposure to Legionella contaminated water aerosol from one CT during an instruction meeting may cause infection in healthy people. Infection was confirmed by serological response to the same Legionella pneumophila serogroup 12 as were the isolates from air around this CT, despite other L. pneumophila (L.p.) serogroups (L.p.1,5,10) from water were isolated with low seroconversion in workers.
The analysis underlined importance of prevention and control of CTs with emphasis on Legionellae surviving and all supportive factors of water with protection of workers. Obligatory registration of CTs and legislation would be proved perspective for public health.
No funding or conflict of interest.
MŠ: Designed the study, analyzed the data, drafted the manuscript; MK: Laboratory water sampling, detection of DNA Legionella by PCR in clinical samples. MF: Processing clinical samples for culture and urine for ELISA. DŠ: Air sampling close to CTs and processing samples. All authors edited the manuscript, reviewed and approved the final version, and agreed to be accountable for all aspects of the work.
Authors' Contributions All listed authors made material contributions to this research.