A 5-year-old boy was diagnosed as having chronic mucocutaneous candidiasis at the age of three months. He showed no improvement despite treatment with two courses of intravenous amphotericin B, 2½ years of oral 5-fluorocytosine, and daily treatment with topical Mycostatin. An immune assessment revealed that he was anergic and had low levels of circulating FCγR+ monocytes. Buccal smears revealed numerous candida and no inflammatory response. On treatment with transfer factor his FCγR+ monocyte count increased from 10 to 429 cells/cumm, his total monocyte count rose from 175 to 1,100 cells/cumm, his candida-induced migration inhibition factor production increased by 36%, and his buccal smears showed infiltration with phagocytic leukocytes. He developed delayed cutaneous hypersensitivity to candida antigen and demonstrated marked improvement in his candidiasis. At age seven he developed adrenal insufficiency and hypoparathyroidism and hence fit the criteria for the diagnosis of autoimmune polyendocrinopathy syndrome type-1 (APS-1).
Transfer factor, Dialyzable leukocyte extract, Chronic mucocutaneous candidiasis, Monocytes, Dendritic cells, Autoimmune polyendocrinopathy syndrome type-1, APS-1, AIRE, APECED, Thymosin, Th17 cells
TF: Transfer Factor; DLE: Dialyzable Leukocyte Extract; LEU: Leukocyte Equivalent Units; PBMC: Peripheral Blood Mononuclear Cells; TLR: Toll-Like Receptor; CMC: Chronic Mucocutaneous Candidiasis; APS-1: Autoimmune Polyendocrinopathy Syndrome Type-1; APECED: Autoimmune Polyendocrinopathy Candidiasis Ectodermal Dystrophy; AIRE: Autoimmune Regulator Gene; DCH: Delayed Cutaneous Hypersensitivity; PHA: Phytohemagglutinin; MIF: Macrophage Inhibition Factor; FcγR: IgG receptor; DC: Dendritic Cell; moDC: Monocyte-Derived Dendritic Cell; Tα1: Thymosin Alpha 1; DNCB: Dinitrochlorobenzene
A 5-year-old boy was diagnosed as having chronic mucocutaneous candidiasis (CMC) at the age of three months. The infection involved his fingernails, the skin of his hands and face, his buccal mucosa, tongue, epiglottis, larynx, lung, and esophagus, and failed to respond to repeated courses of topical Mycostatin, to two courses of intravenous amphotericin B, and to a 2½ year course of oral 5-fluorocytosine. He showed no response to skin testing with candida, trichophyton and mumps antigens and to two attempts at dinitrochlorobenzene sensitization. His B and T cell counts and proliferative responses to phytohemagglutinin and candida antigen were normal, and candida-induced migration inhibition factor (MIF) production by blood lymphocytes was present. He was monocytopenic, and levels of blood monocytes displaying FCγR were diminished. Buccal smears showed numerous candida organisms and no inflammatory cells.
A total of 10 doses (27 billion leukocyte equivalent units) (LEU) of transfer factor (TF) were given over a 2½ year period at two to four-month intervals. Two days after receiving an initial dose of 2.9 billion LEU TF, the blood monocyte count rose from 175 cells/cumm to 1,100 cells/cumm, the percent of FCγR positive cells increased from 5.7% to 39% (control levels were 6%), and there was a 2.4-fold increase in the migration inhibition response to candida antigen (Table 1). Similar results were seen with subsequent doses of TF. Monocyte levels of greater than 700-800 cells/cumm were associated with clinical improvement; relapse occurred when monocyte counts fell to pretreatment levels which occurred on average 67 days after each treatment. Despite increasing TF doses, sustained monocytosis of greater than 1,100 to 1,200 cells/cumm did not occur.
Table 1: Monocyte function. View Table 1
Improvement in monocyte counts and function were associated with clinical improvement (complete resolution of epiglottal, laryngeal, pulmonary and esophageal candidiasis, and marked improvement in oral and cutaneous disease), the establishment of delayed cutaneous hypersensitivity (DCH) to candida antigen, and infiltration of infected buccal mucosa with inflammatory cells (Figure 1). Except for mild erythema at the injection sites, there were no adverse reactions to TF treatment.
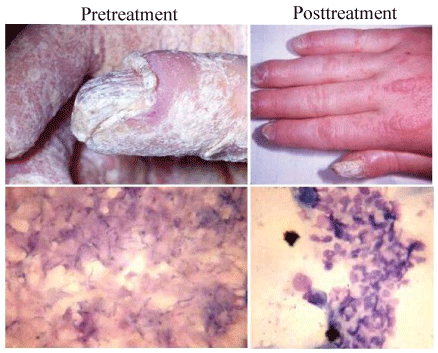 Figure 1: Hand and buccal mucosa photographs taken before and after treatment of the child with TF. Tolerability of the invading candida is evident in the pretreatment sample, as evidence by the total lack of inflammatory response to the organism. Following therapy, clinical improvement was accompanied by marked infiltration of infected buccal mucosal sites by inflammatory cells.
View Figure 1
Figure 1: Hand and buccal mucosa photographs taken before and after treatment of the child with TF. Tolerability of the invading candida is evident in the pretreatment sample, as evidence by the total lack of inflammatory response to the organism. Following therapy, clinical improvement was accompanied by marked infiltration of infected buccal mucosal sites by inflammatory cells.
View Figure 1
The patient eventually developed adrenal insufficiency and hypoparathyroidism and hence fit the clinical criteria for the diagnosis of polyglandular autoimmune syndrome type 1 (APS-1) also referred to as autoimmune polyendocrinopathy candidiasis ectodermal dystrophy (APECED) [1]. This syndrome is now recognized to be due to mutations in the transcriptional regulator and proapoptotic factor, AIRE [2]. AIRE plays an important role in T cell tolerance induction in the thymus, mainly by promoting the expression of ectopic proteins by medullary thymic epithelial cells. The lack of a functional AIRE results in impaired clonal deletion of self-reactive lymphocytes, which are released into the periphery and attack a variety of organs [1,2].
CMC in APS-1 patients has been found to correlate with the production of autoimmune neutralizing antibodies to T-helper-type17 (Th17)-associated cytokines interleukin (IL)-17A, IL-17F, and IL-22 [3,4]; these cytokines, which were not measured in our patient, are known to play an important role in the protection of epithelial surfaces against fungal invasion. Th17 family cytokines protect epithelial surfaces from microbial invasion by prompting innate immune, endothelial, and epithelial cells to produce G-CSF, IL-8 (CXCL8), monocyte chemokines, and GM-CSF thereby upregulating neutrophil and monocyte/macrophage recruitment to sites of infection [5,6].
In 1942 Landsteiner and Chase performed an experiment that was to provide a new paradigm for the field of immunology. They removed peritoneal exudates from guinea pigs previously sensitized intraperitoneally with picryl chloride and conferred DCH to the compound by injecting the washed exudates into the abdominal cavities of skin test negative guinea pigs [7]. They had demonstrated the transfer of DCH by leukocytes, and, in so doing, provided the first convincing evidence of the existence of separate cellular and humoral immune compartments.
In 1949 Dr. Sherwood Lawrence and his associates extended the observations of Landsteiner and Chase by transferring DCH to tuberculin using leukocytes from tuberculin skin-test positive human donors and non-reactive human recipients [8]. They found that the transfer activity resided largely within lymphocytes, and that its activity was rapid in onset and of long duration. They found that leukocyte lysates and dialysates imparted DCH in a manner similar to the intact cell, and that the activity was not abrogated by trypsin, deoxyribonuclease and ribonuclease. They concluded that the active material was a low molecular weight "informational" or "activator" molecule which conferred specific antigen-reactivity to naïve lymphocytes, possibly by effecting gene de-repression. The substance was named transfer factor (TF) [9].
We now know that TF contains more than 200 highly polarized, hydrophilic, low molecular weight peptides, and that it is capable of transferring both specific and non-specific immunity to healthy recipients. TF can be extracted from human and animal white blood cells, cloned lymphocytes grown in vitro, and colostrum, and is capable of functioning across species [10].
Although our case predated the modern era of immunology in which testing of lymphocyte subsets, cytokines, growth factors, and transcriptional regulators has become routine, it is, nonetheless, informative. It confirmed reports of others that high doses of TF can be helpful in treating children with recalcitrant CMC [11-14]. CMC can be divided into four groups based on the extent of skin and mucous membrane involvement and the presence of cutaneous granuloma and/or endocrinopathy. Immunologically, the one invariant finding in this heterogeneous group of diseases is the presence of cutaneous anergy or hypoergy. MIF production in response to candida antigen may or may not be present, and in vitro proliferative responses of lymphocytes to mitogens and candida antigen are usually normal [15].
Our patient had recalcitrant candida infection and polyendocrinopathy and hence fit the clinical criteria for the diagnosis of APS-1 [1]. TF treatment changed his state of complete tolerance to candida infection to one of vigorous intolerance. This coincided with the mobilization and activation of blood monocytes, the invasion of mucosa by inflammatory cells, the establishment of DCH to candida antigen, and clinical improvement. One other case of CMC with defective mononuclear leukocyte chemotaxis has been reported [16].
The precise mechanism(s) whereby TF initiated the immunological changes observed in our patient is not known, but several possibilities are worth considering.
One theory states that blood monocytes and monocyte-derived dendritic cells (moDCs), but not CD4+ lymphocytes or PMN, express the AIRE gene, and that this transcription regulator plays a pivotal role in the maturation of moDCs but is mutated in APS-1 [17]. Studies have shown that APS-1 moDCs have low basal cytokine expression and an impaired ability to mature in response to microbial stimuli [18]. DCs with low levels of antigen responsiveness induce a state of tolerance by causing T cells to become anergic, apoptotic, or Treg, whereas more vigorously responsive DCs upregulate MHC II, adhesion molecules, cytokines, and growth factors, and migrate to local lymph nodes where they initiate adaptive immune responses. Expression of all pattern recognition receptors involved in anti-candida responses (TLR-1, -2, -4, -6, Syk, CARD9, Dectin-1) have been reported to be normal in immature APS-1 moDC [19], suggesting that the maturation defect is not due to lack of receptor recognition, but rather lies in functional pathways involving signal transduction or translation. Hence, it is possible that TF exerts its effect in APS-1 by upregulating the function of these signaling pathways, thereby prompting moDC maturation and subsequent activation of the effector arm of adaptive immunity. Since GMCSF is secreted by DCs (and macrophages), improved function of these cells could also account for the monocytosis seen following TF administration.
In many ways our results parallel those described with thymosin α-1 (Tα1), a pleiotrophic prothymosin polypeptide derivative found in high concentrations in the thymus and in lesser concentrations in other tissues, including peripheral blood mononuclear cells (PBMC) [20]. Synthetic Tα1 has been used to reconstitute several children with APS-1, and to treat some viral infections [21], although seemingly with less efficacy than we found with TF. In humans, Tα1 has been shown to activate immature and mature mDC and pDC subsets, to promote T cell and NK cell maturation, and to stimulate cytokine production and CTL-mediated cytotoxic responses [22-24]. Tα1 has been shown to modulate the expression of a variety of gene transcripts in PBMC in vitro, possible accounting for its pleiotropic effects. Prothymosin α, the precursor of T1α, is a histone H1-binding protein which synergizes with CREB-binding protein to stimulate AP1- and NF-KB-dependent transcription by chromatin remodeling [25]. Whether TF has similar modus operandi in transcription regulation is not known. It is also not known whether TF contains elements of thymic fraction 5, from which Tα1 is isolated; this fraction contains peptides ranging in molecular weight from 1,000 to 15,000 [26] (TF contains peptides with molecular weights of 10,000 or less) (Box 1). Under any circumstance, the conclusion of Lawrence and associates that TF works by "gene de-repression" [5] may well prove to be correct.
Use of transfer factor as a therapeutic agent in this case was approved by the Institutional Review Board of Northshore University Hospital, a past affiliate of Cornell Medical College; the approval included an informed consent signed by his father.